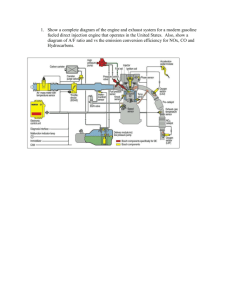
Appendix-C - unix.eng.ua.edu
advertisement
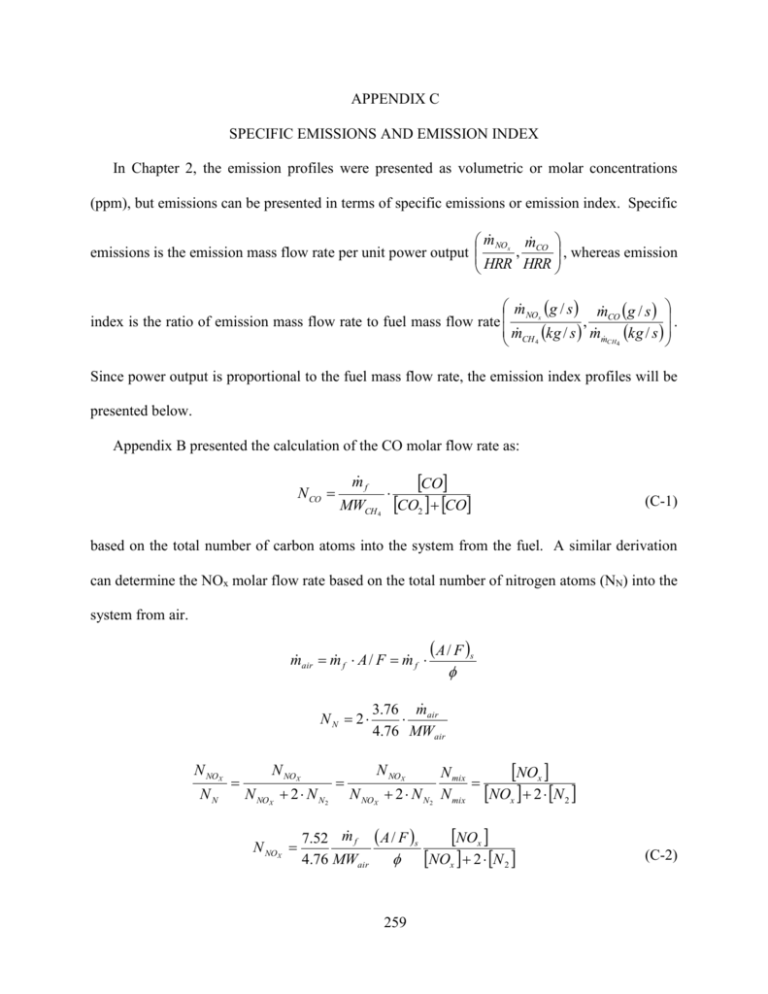
APPENDIX C SPECIFIC EMISSIONS AND EMISSION INDEX In Chapter 2, the emission profiles were presented as volumetric or molar concentrations (ppm), but emissions can be presented in terms of specific emissions or emission index. Specific m NOx m CO , whereas emission , emissions is the emission mass flow rate per unit power output HRR HRR m NO g / s m g / s x . , CO index is the ratio of emission mass flow rate to fuel mass flow rate m CH kg / s m m kg / s 4 CH 4 Since power output is proportional to the fuel mass flow rate, the emission index profiles will be presented below. Appendix B presented the calculation of the CO molar flow rate as: N CO m f MWCH 4 CO CO2 CO (C-1) based on the total number of carbon atoms into the system from the fuel. A similar derivation can determine the NOx molar flow rate based on the total number of nitrogen atoms (NN) into the system from air. m air m f A / F m f NN 2 N NOX NN N NOX N NOX 2 N N2 N NO X A / F s 3.76 m air 4.76 MWair N NOX N NOX 2 N N2 NOx N mix N mix NOx 2 N 2 NOx 7.52 m f A / F s 4.76 MWair NOx 2 N 2 259 (C-2) where concentration of N2 in the dry products is approximated as: [N2] =1-[O2] -[CO2] -[CO] -[NOx] The emission mass flow rates can be computed by multiplying the molar flow rate by the molecular weight of the respective emissions. The primary form of NOx is NO, so the molecular weight of NO is utilized to calculate the NOx mass flow rate. The transverse profiles for NOx concentration and EINOx shown in Figures C-1(a) and (b), respectively, are proportional and demonstrate similar trends of increasing magnitudes as m f decreases in both Figures. The transverse profiles of CO concentration in Figure C-2(a) and EICO in Figures C-2(b) at constant ϕ both demonstrate similar trends of increasing magnitudes as m f decreases. With constant ϕ, the NOx concentration in Figure C-1(a) and CO concentration in Figure C-2(a) are not affected by changes in excess air. Thus, the similar profiles between the concentrations and emission indexes were expected. a) b) Figure C-1. Transverse a) NOx concentration and b) EINOx at the combustor exit plane, z = 21 mm; = 0.70. 260 a) b) Figure C-2. Transverse a) CO concentration and b) EICO at the combustor exit plane, z = 21 mm; = 0.70. The transverse profiles of NOx concentration in Figure C-3(a) and EINOx in Figures C-3(b) show slight variations due to the different calculations. However, both Figure C-3(a) and (b) demonstrate NOx concentrations and EINOx increase as ϕ increases. The difference between the transverse profiles of CO concentrations in Figure C-4(a) and EICO in Figure C-4(b) demonstrate a more significant effect due to the different calculations. While the profiles show similar trends of increasing CO concentration and increasing EICO with increasing ϕ, the increase in CO concentrations is larger, proportionally, than the increase in EICO. The indication is that the increase in excess air with decreasing ϕ is partially responsible for the decrease in CO concentrations. The increase in EICO as ϕ increases could be the result of increasing product gas temperature resulting in dissociation of CO2 or a reduction in available oxidizer resulting in a decrease of CO oxidation. 261 a) b) Figure C-3. Transverse a) NOx concentration and b) EINOx at the combustor exit plane, z = 21 mm; m f = 20 g/hr. a) b) Figure C-4. Transverse a) CO concentration and b) EICO at the combustor exit plane, z = 21 mm; m f = 20 g/hr. 262