Stoichiometry & Reaction Classification
advertisement

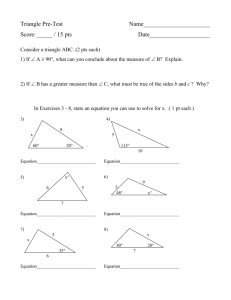
Summer 2009 Chem 161 Version A Exam 2 (100 pts) July 13, 2009 Name______________________ Section A B 1. (10 pts) Analysis of nicotine shows that it is 74.0% C, 8.65% H, and 17.35% N. Its molar mass is 162 g/mol. What are the (a) empirical formula and (b) molecular formula of nicotine? 2. (8 pts) Assign an oxidation number to chlorine in each of the following anions. For c and d, give chemical formulas first then assign the oxidation number. a) ClO- b) ClO2- c) chlorate _________ d) perchlorate ________ 3. Using the activity series, predict whether the following reactions should occur. If so, provide products and balance the equation. If not, then state “no reaction”. (8 pts) a) Ca(NO3)2 (aq) b) Cu (s) + c) SnCl2 (aq) + Mg (s) HCl (aq) + Zn (s) 4. (12 pts) A solution is prepared by dissolving 25.0 g of (NH4)SO4 in enough water to make 100.0 mL of stock solution. A 10.00-mL sample of this stock solution is added to 50.00 mL of water. Calculate the concentration (molarity) of the: a) ammonium sulfate solution (b) ammonium ions and (c) sulfate ions in the final solution. 5. In the juice lab, you performed a redox titration to determine the amount of ascorbic acid in juice. You used a solution of KIO3 for the titration. (14 pts) a) Write an equation for the dissociation of KIO3 into two ions (include charges): KIO3 (aq) Iodine is formed from the use of potassium iodate in this chemical equation: IO3-(aq) + 5I- (aq) + 6H+(aq) 3I2 (aq) + 3H2O(l) b) Assign oxidation numbers to only iodine-containing species in this equation. c) List reactant(s) oxidized in this reaction. __________________________ d) List reactant(s) reduced in this reaction. _________________________ The iodine formed then reacts with ascorbic acid in the following equation: C6H8O6 (aq) + I2 (aq) ascorbic acid C6H6O6(aq) + 2I-(aq) + 2H+(aq) e) Does ascorbic acid get oxidized or reduced in this equation? ______________ f) Is the iodine the oxidizing agent or reducing agent in this reaction? ____________ 6. (8 pts) Predict the products from the following reactions and complete/balance the equations if a reaction occurs. If no reaction occurs, write “no reaction”. a) HCl (aq) + Ca(OH)2 (aq) b) HCl (aq) + CaCO3 (aq) c) BaCl2 (aq) + H2SO4 d) CuSO4 (aq) + HNO3 (aq) 7. Aqueous solutions of silver nitrate and calcium chloride are mixed. a) Write a balanced molecular, complete ionic, and net ionic equation for the reaction that occurs. INCLUDE PHYSICAL STATES (aq), (s), (g), (l) in all three equations! (12 pts) Molecular: Complete Ionic: Net Ionic: b) How many grams of precipitate can be prepared by the reaction of 3.398 g of silver nitrate (169.9 g/mol) with 1.6647 g of calcium chloride (110.98 g/mol)? CIRCLE YOUR ANSWER. (14 pts) c) (4 pts) List the ions that remain in solution. _________________________ d) BONUS QUESTION for the problem above (5 pts): Suppose the total volume of the solution above is 200 mL. What is the concentration (molarity) of ions that remain in solution? CIRCLE YOUR ANSWERS. (Please show your work for the bonus question). NOT FOR BONUS: 8. (10 pts) Your supervisor wants to see if you can figure out the identity of a white powder. To be nice, she says that the empirical formula is C3H4O3. You know that it is acidic because your litmus paper turns pink in a solution of the white powder. You decide to use an acid-base titration to figure out what the compound is. You take 2.20-g sample of the unknown acid and dissolve it in 1.0-L of distilled water, adding a few drops of indicator. The titration requires 25.0 mL of 0.500 M NaOH to reach the endpoint. Assuming that the unknown acid has one acidic proton per molecule, what is the molecular formula of the unknown acid? (No points for guessing—you must show your answer as a “proof”).