Addendum
advertisement
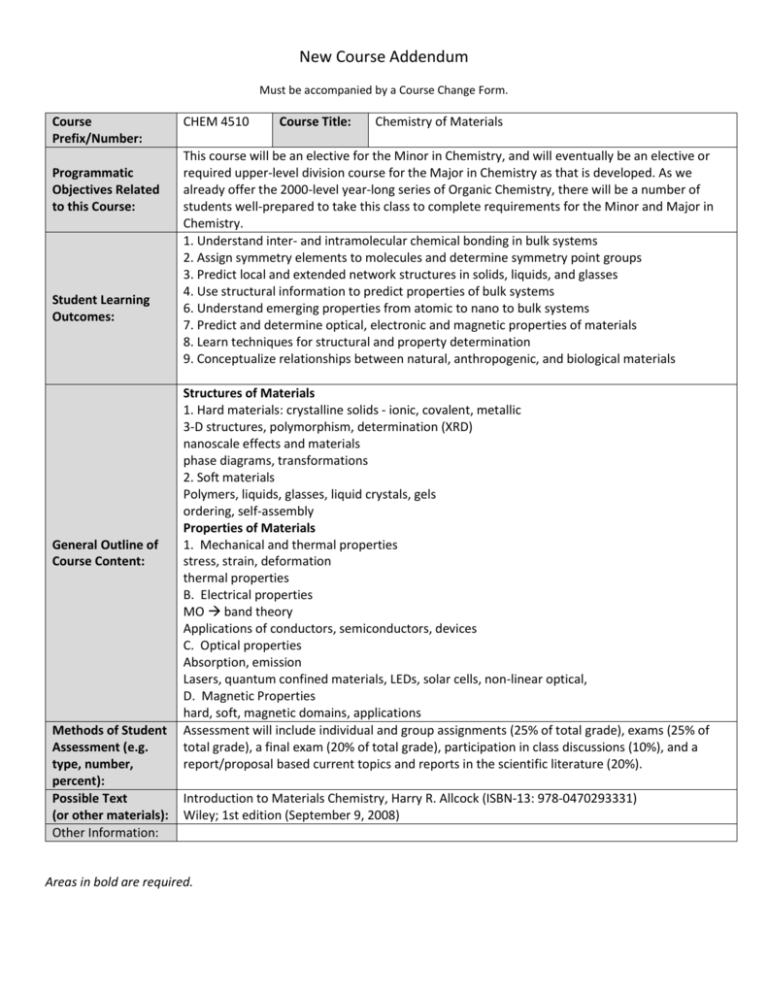
New Course Addendum Must be accompanied by a Course Change Form. Course Prefix/Number: Programmatic Objectives Related to this Course: Student Learning Outcomes: General Outline of Course Content: Methods of Student Assessment (e.g. type, number, percent): Possible Text (or other materials): Other Information: CHEM 4510 Course Title: Chemistry of Materials This course will be an elective for the Minor in Chemistry, and will eventually be an elective or required upper-level division course for the Major in Chemistry as that is developed. As we already offer the 2000-level year-long series of Organic Chemistry, there will be a number of students well-prepared to take this class to complete requirements for the Minor and Major in Chemistry. 1. Understand inter- and intramolecular chemical bonding in bulk systems 2. Assign symmetry elements to molecules and determine symmetry point groups 3. Predict local and extended network structures in solids, liquids, and glasses 4. Use structural information to predict properties of bulk systems 6. Understand emerging properties from atomic to nano to bulk systems 7. Predict and determine optical, electronic and magnetic properties of materials 8. Learn techniques for structural and property determination 9. Conceptualize relationships between natural, anthropogenic, and biological materials Structures of Materials 1. Hard materials: crystalline solids - ionic, covalent, metallic 3-D structures, polymorphism, determination (XRD) nanoscale effects and materials phase diagrams, transformations 2. Soft materials Polymers, liquids, glasses, liquid crystals, gels ordering, self-assembly Properties of Materials 1. Mechanical and thermal properties stress, strain, deformation thermal properties B. Electrical properties MO band theory Applications of conductors, semiconductors, devices C. Optical properties Absorption, emission Lasers, quantum confined materials, LEDs, solar cells, non-linear optical, D. Magnetic Properties hard, soft, magnetic domains, applications Assessment will include individual and group assignments (25% of total grade), exams (25% of total grade), a final exam (20% of total grade), participation in class discussions (10%), and a report/proposal based current topics and reports in the scientific literature (20%). Introduction to Materials Chemistry, Harry R. Allcock (ISBN-13: 978-0470293331) Wiley; 1st edition (September 9, 2008) Areas in bold are required.