the Template here - SBQT-2015
advertisement

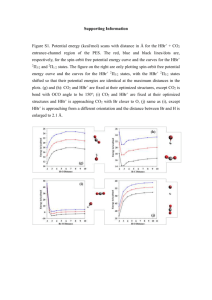
XVIII Simpósio Brasileiro de Química Teórica – SBQT 2015 Pirenópolis – GO, 22-25 Novembro de 2015 On the Origin of the Negative Activation Energy in the OH+hbr Reaction Rate Constant: Born-Oppenheimer Molecular Dynamic Study of Non-Arrhenius Behavior Author name who will present the work a (PQ or PG), Second Authorb (PQ or PG) a b Address 1 Address 2 Keywords: Ab Initio Molecular Dynamic, OH + HBr Reaction, Non-Arrhenius, Negative Activation Energy INTRODUCTION In recent years several studies of gas-phase reactions, which are believed to be elementary, have been shown to present either curved Arrhenius plots1 or even negative activation energies.2 The reaction of the OH radical with HBr molecule is a relevant example of an elementary process presenting negative activation energy from Arrhenius plots of the measured temperature dependence. The apparent negative activation energy of the reaction rate constants shows up below 200K. For these reasons many theoretical studies have been published to explain this unusual behavior2 In this work we used Born-Oppenheimer molecular dynamics for understanding and to explain the appearance of a negative activation energy for the OH+ + HBr reaction. Accordingly, the manifestation of a negative activation energy observed in this reaction is attributed to the orientation between vdW complex formation and the molecular orientation between reactants. Conversely, at high energies the molecules fail to orient themselves and disfavor the formation of the complex. Figure 1. Effective free-energy profile for the OH• + HBr reaction: a) lower temperature, b) higher temperature. METHODS CONCLUSIONS The Born-Oppenheimer molecular dynamics method was used to study OH+ + HBr reaction. Periodic boundary conditions, norm conserving pseudopotentials of the Troullier_Martins and functional by Perdew, Burke and Ernzerhof were employed. The free energy profile was obtained from ∆𝐺(𝑉) = −𝑘𝐵 𝑇𝑙𝑛[∫ 𝑃(𝑅, 𝑉)𝑑𝑅], where the coordinates 𝑉 and 𝑅 were defined on the basis of the Jacobi’s vector scheme. The simulations performed in this work reveal the origin of the negative curvature on the OH+ + HBr reaction rate constant, in agreement with the experiments. A key role is played by the vdW complex formation, strongly dependent on the effectiveness of molecular alignment. Therefore, the dynamics presents two limiting pathways, characterizing the low and high temperature behavior of this reaction, which is influenced by the effectiveness of the vdW complex. RESULTS AND DISCUSSION During the simulations, we found two reactions pathways: (i) At lower energies, only the direct hydrogen abstraction from HBr through Van der Waals (vdW) complex and the transition state has lower energy than the reactants (Fig.1a); (ii) At higher energies, there is no formation of vdW complex and the transition state energy has higher energy than the reactants (Fig.1b). This result seems to be consistent with recent crossed molecular beam studies of the steric effect for this reaction.3 ACKNOWLEDGMENTS The authors are grateful for the support given from the FAPEG, CAPES, CNPQ and FINATEC. 1 S. W.Benson and O. Dobis, J. Phys. Chem. A,102 , 5175, (1998). 2 J. Liu, Z.Li, C.Sun, J. Phys. Chem. A, 105, 7707, (2001). 3Tsai, P.-Y.; Che, D.-C.; 3 M. Nakamura, K. C. Lin, T. Kasai, Phys. Chem. Chem. Phys.,12, 2532,(2010).