Method - ChemistryShiok
advertisement

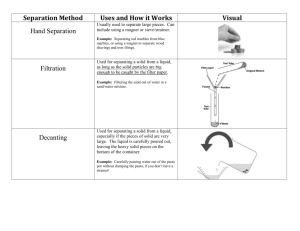
Chapter 2 Methods of Purification and Analysis Name: ___________________ Class: ________ ( ) Date: ________________ Lesson Aims Describe methods of separation for the components of the following types of mixtures: (i) solid-solid (ii) solid-liquid (iii) liquid-liquid(miscible) Techniques to be covered for separations and purification include: (i) Use of a suitable solvent, filtration and crystallization or evaporation (ii) Distillation and fractional distillation (iii) Paper chromatography Describe paper chromatography and interpret chromatograms. Identify substances and test their purity by melting point and boiling point determination and by paper chromatography. 2.1 What is a pure substance? A pure substance contains only one type of substance, and is not mixed with any other substance. 2.2 Separating SOLID – LIQUID 1) Filtration (Insoluble solid – liquid) Filtration is used to separate an insoluble solid from a solid – liquid mixture. The filter paper acts as a sieve, which allows the smaller liquid particles to pass through and keeps the bigger solid particles behind. r______________ – the insoluble solid trapped in the filter paper f______________ – the liquid that passes through the filter paper 2) Decanting (Insoluble solid – liquid) Decanting is used to separate an insoluble solid from a solid – liquid mixture. Liquid is carefully poured away from the solid, which is usually heavier and settles at the bottom of the container. 3) Evaporation to Dryness (Soluble solid – liquid) It is used to recover a soluble solid from its solution. Only for solids that will NOT decompose on heating. An example is heating salt solution to dryness to recover the sodium chloride (salt) left behind. 4) Crystallization (Soluble solid – liquid) It is used to recover a soluble solid from its solution. For solids that will decompose on heating. An example is copper(II) sulfate and most other salts. Steps: a) The solution is heated (evaporated) to saturation point OR ‘heated to remove most of the solvent’ b) The saturated solution is left to cool; crystals are formed. c) The crystals are removed by filtration. To purify the crystals, they can then be washed with cold distilled water and dried between filter papers. This method works because substances have higher solubility in hot solvent than in cold solvent. When the hot saturated solution is cooled, the solubility of the solute decreases. The extra solute that cannot remain dissolved appears as crystals. 2.3 Separating SOLID – SOLID Method used depends on the nature of the solids: By Dissolution If the mixture of solids behaves differently in a particular solvent, that is, one component is soluble in it while the other is insoluble… Choose a solvent that will dissolve only ONE of the solids By Sublimation It is used when one of the solids sublimes. Heat the solid – solid mixture and one of the solids will turn into vapour. Collect the sublimate after it condenses. Examples: Mixture of iodine (sublimes) and copper Mixture of ammonium chloride (sublimes) and sodium chloride By Other Properties (Magnetism) Make use of the property of one of the solids. Example: Mixture of iron filings and sulfur powder Bring a magnet to the mixture, attract the iron filings and sulfur powder will be left behind. 2.4 Recovering the SOLVENT in a solution (Distillation) Principle behind Distillation A liquid boils and turns into vapour at its boiling point. When the vapour is condensed, the (pure) liquid is obtained again. Simple Distillation (eg. sea water) Liquid that is distilled over is called DISTILLATE. The remaining solid is called residue. Direction of Water Flow in Condenser Water flows in the opposite direction to the flow of vapour. This is to make sure the coldest part of the condenser is just before the vapour escapes. Position of Thermometer The thermometer is placed at the same level as the side – arm, so that it measures the temperature of the vapour as it enters the condenser. Other Examples of Simple Distillation Water from copper (II) sulfate solution Ether from a solution of sugar in ether 2.5 Separating LIQUID – LIQUID (Fractional Distillation) Fractional distillation is used to separate MISICIBLE liquids. Miscible liquids are liquids that mix completely to form a single layer. Principle behind Fractional Distillation Different liquids have different boiling point. The liquid with the lower boiling point turns into vapour first. The vapour rises and enters the fractionating column and condensation takes place in it. Some of the vapour enters the condenser and is distilled over. Vaporization and condensation occurs many times. When all of the first liquid has been distilled over, the temperature of the remaining liquid in the flask will continue to rise until it reaches the boiling point of the second liquid. The second liquid turns into vapour at boiling point is distilled over. Its distillate is collected separately. Things to Note The fractionating column consists of glass beads that provide a large surface area so that condensation can occur more readily. The liquid with lower boiling point will distil over first followed by the liquid with higher boiling point. Temperature-time Graph Sketch the temperature time graph of a mixture of water (b.p. 100 oC) and ethanol (b.p 78 oC) undergoing fractional distillation. Other Examples of Fractional Distillation Nitrogen and oxygen in liquid air Components of crude oil Ethanol from fermented liquor 2.6 Separating Immiscible Liquids (Separating Funnel) Immiscible liquids are liquids that do not mix, eg. water and oil. A separating funnel can be used to separate two immiscible liquids. (a) The mixture is placed in the separating funnel and allowed to settle, until two layers are formed. (b) Open the tap. Allow the bottom layer to run into a beaker. Close the tap (c) The top layer can then be run into a separate beaker. 2.7 Chromatography Chromatography is a method of separating and identifying mixtures, eg. to identify artificial dyes in food. The advantage is that only a small amount of the mixture is needed for testing. Principle behind Chromatography The most soluble solute will be carried quickly up the paper with the solvent while the least soluble solute will be left behind. The start line is drawn with pencil to prevent because pen ink will dissolve in the solvent. The spots are above the solvent and the solvent just touches the tip of the paper. Spots should be small so that when it dissolves it will not affect the other spots beside it. Locating reagent If the spots are colourless, the dried chromatogram has to be sprayed with special locating reagent which shows up the components as coloured spots. Uses of Chromatography Separate (a) pigments from plants, (b) dyes from inks and (c) amino acids from proteins for analysis Identify poisons and drugs or illegal dyes or addictives in foodstuff Determine purity of a given substance 2.8 Criteria for Purity Pure substance - substance with no other substances mixed with them - has fixed (sharp) melting point and boiling point Testing the purity of a substance: Method 1. Checking its melting point 2. Checking its boiling point v Impurities will lower the melting point and raise the boiling point. NOTE: No two substances have the same pair of melting and boiling pts. v For an impure substance, the temperature Hence melting and boiling pts. can be does not stay constant during used to identify a substance. boiling/melting. 3. Using Chromatography v A pure substance will give only one spot in the chromatogram.