File
advertisement

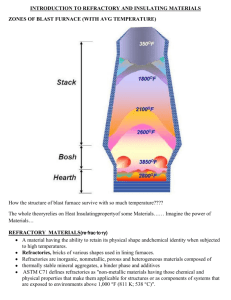
Lowell D. Outland IET 307 Materials Science 307: Materials Science HW 7 (based on chapters 12 & 13), Due on November 6'Th (Sunday, before 11.55 PM), 2011, 100 points (Cite external references wherever used) 1. What are the AX-Type crystal structures? Explain the rock salt structure, cesium chloride structure & zinc blende structure in a few sentences and then draw a schematic of each structure. (10 points) AX-type Crystal structures are those that have an equal number of cations and anions, where A denotes the cation (metallic ions) and X denotes the anion (nonmetallic ions). Each of the materials are normally named after a common material that assumes the particular structure. Rock Salt Structure or sodium chloride (NaCi) is perhaps the most common AX crystal structure. The coordination number for both cations and anions is 6, and therefore the cation-anion ratio is between approximately 0.414 and 0.732. a unit cell for this crystal structure is generated from an FCC arrangement of anions with one cation situated at the cube center and one at the center of each of the 12 cube edges. An equivalent crystal structure results from a face centered arrangement of cations. Thus the rock salt crystal structure may be thought of as two interpenetrating FCC lattices, one composed of cations, the other of anions. Image retrieved from http://static.newworldencyclopedia.org Cesium Chloride Structure – The coordination number for both ion types is 8. The anions are located at each of the corners of a cube, whereas the cube center is a single cation. Interchange of anions with cations, and vice versa, produces the same crystal structure. This is not a BCC crystal structure, because ions of two different kinds are involved. Lowell D. Outland IET 307 Materials Science Image Retrieved from www.nrl.navy.mil Zinc Blende structure or sphalerite – The coordination number is 4; that is all ions are tetrahedrally coordinated. All corner and face positions are of the cube cell are occupied by S atoms, whereas the Zn atoms fill the interior tetrahedral positions. An equivalent structure results if the Zn and S positions are reversed. Thus each Zn atom is bonded to 4 S atoms and vice versa. Most often the atomic bonding is highly covalent in compounds exhibiting this crystal structure, which include ZnS, ZnTe, and Sic. Image retrieved from http://www.chm.bris.ac.uk 2. Explain in a few sentences for each of the following ceramic materials, their properties and applications (10 points). (a) Lead zirconium titanate – This ceramic exhibits the phenomenon of piezoelectricity and is used in such applications as transducers, sonar and other sensors. (b) Cordierite – commonly used as a gemstone (c) Barium titanate – this material falls into the category of AmBnXp crystal structure, because it has both Ba2+ and Ti4+ cations. (d) Zirconia – this material can have three different crystal structures, tetragonal, Lowell D. Outland IET 307 Materials Science monoclinic, and cubic. Pure ZrO3 experiences a tetragonal to monoclinic transformation at about 11000 C. A rather large volume change accompanies this transformation, which results in the formation of cracks that render the ceramic wares useless. This can be remedied by adding 3 to 7 wt% CaO, which stabilizes the material. One application is the use of this material in jewelry. 3) What material is used to make the tiles that provide protection is NASA’s space shuttle? What properties of this material enable it to be used at very high temperatures? Why is the tensile strength of ceramics much lower than that under a compressive stress? (10 points) Much of the shuttle is covered by LI-900 silica tiles (according to wikipedia), made of very pure quartz sand. These tiles are such poor conductors of heat that you can hold one while it is still red hot. For tensile stress there exists a number of stress amplifiers that act upon minute surface and interior flaws within the material to cause the material to fracture. While under compression stress there are no stress amplifiers associated with existent flaws. 4) On the basis of crystal structure, compute the theoretical density for manganese sulphide (MnS). How does this compare with its measured density? (Hint: See example problem 12.3 on page 463 of your text book) (10 points). 5) Discuss the various polymorphic forms of carbon and its properties. 10 points) Diamond- a metastable carbon polymorph at room temperature and atmospheric pressure. Its crystal structure is a variant of zinc blende in which carbon atoms occupy all positions. The physical properties of a diamond are 1) it is extremely hard (the hardest known material), 2) it has very low electrical conductivity. These characteristics are due to its crystal structure and strong interatomic covalent bond. Lowell D. Outland IET 307 Materials Science Graphite – has a crystal structure distinctly different from that of a diamond, and also more stable than a diamond at ambient temperature and pressure. It is composed of layers of hexagonally arranged carbon atoms. It has relatively high electrical conductivity, and excellent lubricative properties. Other properties include: high strength and good chemical stability at elevated temperatures, high thermal conductivity, good absorption of gases and good machinability. Fullerenes - Discovered in 1985 it exists in discrete molecular form and consists of a hollow spherical cluster of sixty carbon atoms. A single molecule is denoted by C60. This material can be made highly conductive and semi-conductive. 6) Discuss micro-electro-mechanical systems (MEMS) and why ceramics play an important role in these systems. (10 points) MEMS are miniature smart systems consisting of a multitude of mechanical devices that are integrated with large numbers of electrical elements on a silicon substrate. These components are micro-sensors and micro-actuators. Then micro-sensors collect environmental information by measuring mechanical, thermal, chemical, optical, and/or magnetic phenomena. The micro-electric components then process the sensory input and render decisions that direct responses from the micro-actuator devices. These are important because of the reliability and economic cost of production, also because they can be produced in such miniscule size, the applications for such products are endless, especially perhaps in the medical field. 7) What is the role of Ytlria stabilized coating (YSZ) on certain turbine blades? Why does the zirconia have to be stabilized? Give examples of some ceramic materials that are used to make lasers? (10 points) An yttria stabilized coating is to protect the blades from extreme heart, this coasting is used as a heat barrier. Pure ZrO3 experiences a tetragonal to monoclinic transformation at about 11000 C. A rather large volume change accompanies this transformation, which results in the formation of cracks that render the ceramic wares useless. This can be remedied by adding 3 to 7 wt% CaO, which stabilizes the material. One application is the use of this material in jewelry. Lowell D. Outland IET 307 Materials Science One ceramic material that is used to make lasers would be Alumina, another is glass(noncrystalline ceramics). 8) What are some of the important properties of sintered ceramics? Explain the powder pressing process? (10 points) Some important properties of sintered ceramics are; 1) a reduction in porosity, and 2) an improvement in mechanical integrity. In powder pressing the die is filled with powder, then the powder is compacted with pressure from the top die. The compacted piece is ejected by the rising action of the bottom punch. Then the fill shoe pushes away the compacted piece and the process starts over. 9) Explain hydroplastic forming & slip casting in a few sentences and then draw schematics of both the process. (10 points) Hydroplastic forming is the shaping or forming of clay-based material that have been made plastic and pliable by the addition of water. Slip casting is a forming technique that is used for some ceramic materials. A slip or suspension of solid particles in water is poured into a porous mold. A solid layer forms on the inside wall as water is absorbed by the mold, leaving a shell and ultimately a solid piece having the shape of the mold. 10) What are refractories? Discuss the various types of refractrories. (10 points) Refractories are a class of ceramics that have the ability to withstand extremely temperatures without decomposing or melting, and the capacity to remain unreactive and inert when exposed to severe environments. Lowell D. Outland IET 307 Materials Science Fireclay refractories – the primary ingredients for fireclay refractories are high purity fireclays, alumina and silica mixtures usually containing between 25 and 40 wt% alumina. According to the SiO2-Al2O3 phase diagram, over this composition range the highest temperature possible without the formation of a liquid phase is 1587oC. below this temperature the equilibrium phases present are mullite and silica (cristobalite ). During refractory service use the presence of a small amount of a liquid phase may be allowable without compromising the mechanical integrity. Upgrading the alumina content will increase the maximum service temperature, allowing a small amount of liquid. Silica Refractories – the prime ingredient of silica refractories, sometimes called acid refractories, is silica. These materials well known for their high-temperature load-bearing capacity, are commonly used in arched roofs of steel and glass making furnaces; for these applications temperatures as high as 1650oC may be realized. The presence of even a small amount of alumina has an adverse influence on the performance of these refractories. Basic Refractories – these refractories that are rich in periclase, or magnesia, are termed basic; they may also contain calcium, chromium and iron compounds. The presence of silica is deleterious to their high temperature performance. Basic refractories are especially resistant to attack by slags containing high concentrations of MgO and CaO and find extensive use in some steel making open hearth furnaces. Special refractories – other ceramic materials are used for rather specialized refractory applications. Some of these are relatively high purity oxide materials. Many of which may be produced with very little porosity. Included in this group are alumina, silica, magnesia, beryllia, zirconia, and mullite. Others include carbide compounds in addition to carbon and graphite. Silica carbide has been used for electrical resistance heating elements, as a crucible material, and in internal furnace components. Carbon and graphite are very refractory, but find limited application because they are susceptible to oxidation at temperatures in excess of 800oC. as would be expected these specialized refractories are relatively expensive.