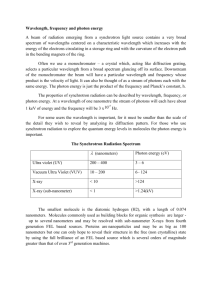
Kilometer km 1 km = 103 m radio Megahertz Hz 1 megahertz = 1.0 x
advertisement
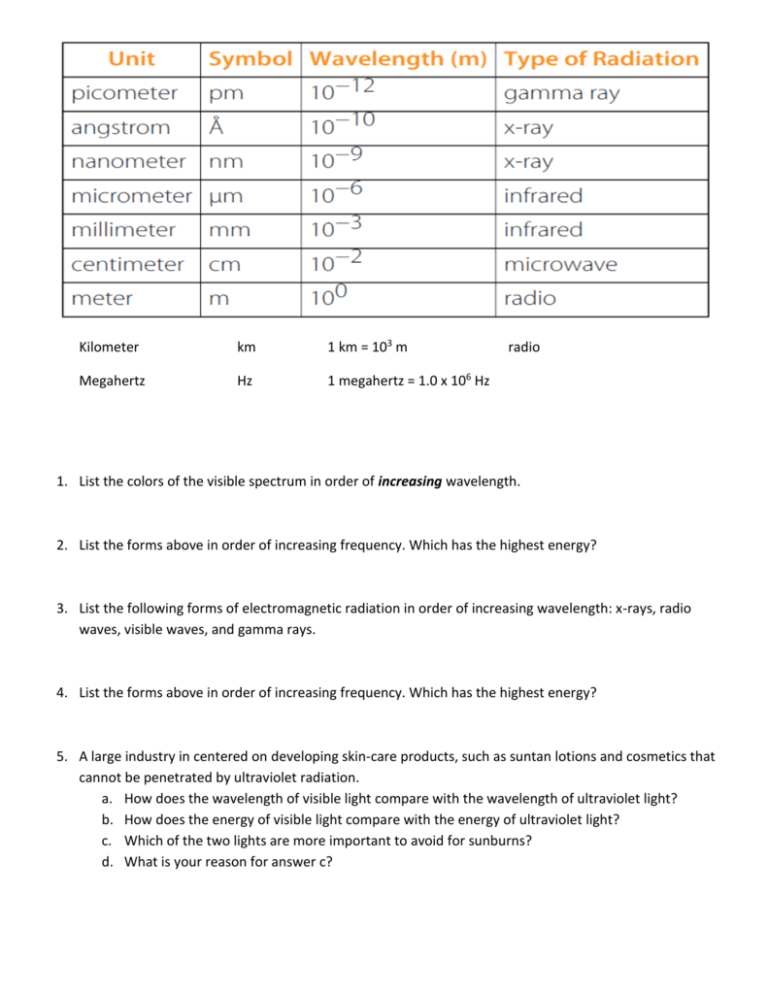
Kilometer km 1 km = 103 m Megahertz Hz 1 megahertz = 1.0 x 106 Hz radio 1. List the colors of the visible spectrum in order of increasing wavelength. 2. List the forms above in order of increasing frequency. Which has the highest energy? 3. List the following forms of electromagnetic radiation in order of increasing wavelength: x-rays, radio waves, visible waves, and gamma rays. 4. List the forms above in order of increasing frequency. Which has the highest energy? 5. A large industry in centered on developing skin-care products, such as suntan lotions and cosmetics that cannot be penetrated by ultraviolet radiation. a. How does the wavelength of visible light compare with the wavelength of ultraviolet light? b. How does the energy of visible light compare with the energy of ultraviolet light? c. Which of the two lights are more important to avoid for sunburns? d. What is your reason for answer c? 6. An AM radio station broadcasts with a wavelength of 248.0 m. What is the broadcast frequency of the station in Hz? 7. Suppose your favorite AM radio station broadcasts at a frequency of 1150 kHz. 1 kHz = 1000 Hz. What is the wavelength in m? 8. The red color seen in fireworks is usually due to the emission of light with wavelengths around 650 nanometers when strontium (Sr – Atomic Number 38) salts are heated. Calculate the frequency, in Hz, of red light of wavelength 6.50 x 102 nm. 1 nm = 1.0 x 10-9 m. 9. A laser dazzles the audience in a rock concert by emitting green light with a wavelength of 515 nanometers. Calculate the frequency of the light in Hz. 1 nm = 1.0 x 10-9 m. 10. Calculate the wavelength, in meters, of the yellow light emitted by a sodium lamp if the frequency of the light is 5.10 x 108 megahertz. 1 megahertz = 1.0 x 106 Hz. 11. The red barcode scanner found at grocery stores has a frequency of 4.62 x 108 megahertz. 1 megahertz = 1.0 x 106 Hz. Calculate the wavelength of the red laser in nanometers. 1 nm = 1.0 x 10-9 m. 12. An FM radio typically has a broadcast range between 82 MHz and 112 MHz. What are the wavelengths, in meters, for both frequencies? 13. An FM radio station broadcasts with a wavelength of 3.21 m. What is the broadcast frequency of the station in hertz? 14. What is the wavelength in nanometers of wave with a frequency of 4.46 x 1010 Mhz? 1 MHz = 1,000,000 Hz. 15. A mercury lamp emits radiation with a wavelength of 4.36 x 10-7m. a. What is the wavelength in nano-meters? b. In what color/area of the electromagnetic spectrum is this energy? c. Calculate the frequency in Hz A hydrogen lamp emits several different waves. One of these waves has a wavelength of 6.56 x 10 -5 cm. What are the color and the frequency, in Hz, of this radiation? 16. A microwave oven operates at a frequency of approximately 2450 Mhz. What is the corresponding wavelength? Water, with its polar molecules, absorbs electromagnetic radiation primarily in the infrared portion of the spectrum. Given this fact, why are microwave ovens used for cooking food? 17. What is the frequency in megahertz corresponding to each wavelength? a. 1.77 x 103km e. 2.3 angstrom b. 9.88 angstrom f. 8.6 x 107m c. 3.7 x 10-10 m g. 6.2mm -7 d. 5.8 x 10 m h. 3.7 nm -1 18. What is the frequency, in sec , for a wave that has a wavelength of 5.00 x 10-6cm? In what region of the electromagnetic spectrum is this radiation? 19.