Pre CH17 HW for Silberberg (a) Define equilibrium. (b) Write
advertisement

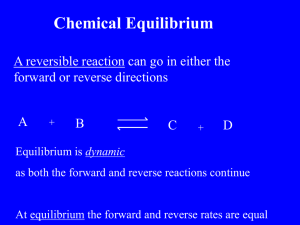
Pre CH17 HW for Silberberg (1) (a) Define equilibrium. (b) Write equilibrium constant (K) expression for the equation…… Ag+(aq) + 2 NH3(aq) Ag(NH3)2+(aq) 2 NOBr(g) 2 NO(g) + Br2(l) (c) If the reaction has K value bigger than 1, what does it mean? (d) What does the letter I, C, and E stand for in ICE table? (e) List one difference between Kc and Kp. List one similarity between Kc and Kp. (f) List one difference between Q and K. List one similarity between Q and K. (g) Given A B has H = -10.kJ, H for B A should be _______, and H for 3A 3B should be ______. (h) Given A B has , for B A should be _________, and for 3A 3B should be ________. (i) Rewrite the quadratic formula. • At the end of each section of the chapter, you will find “Summary of Section x.x” . For example, the Summary of Section 17.1 is on page 733. Please re-write this for section 17.1 to 17.6. You may copy it exactly or use your own words. Clearly label each section. Do this on separate piece of paper and attach to this sheet. • At the end of the chapter, there is a section called “Key Equations and Relationships”. For each section, re-write the important point. If it includes mathematical relationship, name the equation and define each term. Be as specific as possible and include the common units if known. Do this on separate piece of paper and attach to this sheet. • There are 15 “ Sample Problems” (Sample Problem 17.1 to Sample Problem 17.15) all throughout the chapter. Carefully read them all. Then, pick TWO of your choice and re-write the Problem, Plan, Solution and Check. Clearly label the Sample Problem number. Do this on separate piece of paper and attach to this sheet.