What Do We Know About Fireworks?
advertisement
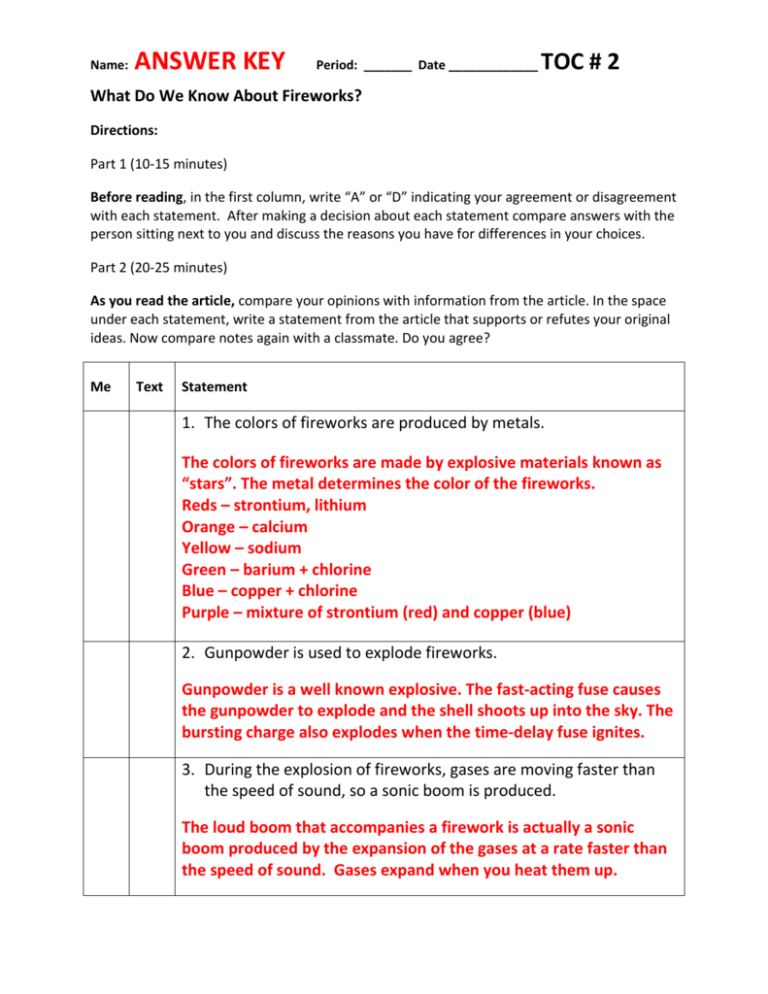
Name: ANSWER KEY Period: _______ Date _____________ TOC # 2 What Do We Know About Fireworks? Directions: Part 1 (10-15 minutes) Before reading, in the first column, write “A” or “D” indicating your agreement or disagreement with each statement. After making a decision about each statement compare answers with the person sitting next to you and discuss the reasons you have for differences in your choices. Part 2 (20-25 minutes) As you read the article, compare your opinions with information from the article. In the space under each statement, write a statement from the article that supports or refutes your original ideas. Now compare notes again with a classmate. Do you agree? Me Text Statement 1. The colors of fireworks are produced by metals. The colors of fireworks are made by explosive materials known as “stars”. The metal determines the color of the fireworks. Reds – strontium, lithium Orange – calcium Yellow – sodium Green – barium + chlorine Blue – copper + chlorine Purple – mixture of strontium (red) and copper (blue) 2. Gunpowder is used to explode fireworks. Gunpowder is a well known explosive. The fast-acting fuse causes the gunpowder to explode and the shell shoots up into the sky. The bursting charge also explodes when the time-delay fuse ignites. 3. During the explosion of fireworks, gases are moving faster than the speed of sound, so a sonic boom is produced. The loud boom that accompanies a firework is actually a sonic boom produced by the expansion of the gases at a rate faster than the speed of sound. Gases expand when you heat them up. Me Text Statement 4. Colored light can be produced when electrons change energy levels inside atoms. Atoms have electrons inside them. When they absorb energy from heat it causes them to move even faster into an “excited” state. They then move to a Lower energy state and emit (give off) light of a specific color. 5. Firework explosions can be designed to look like flowers or trees in the sky. If fireworks are packed carefully in predetermined patterns, then the firework will have a specific shape – such as a flower or a tree – because the “stars” are sent in specific directions during the explosion. 6. Fireworks that can be legally purchased in most states contain less than 10 mg of gunpowder. Forty one of our 50 states allow for the purchase of fireworks. These should have NO more than 50 mg of gunpowder. 7. The highest temperatures produced by firecrackers are only about 500 C. Firecrackers can burn up to temperatures of 1000 degrees Celsius OR 1800 degrees Fahrenheit. Me Text Statement 8. Chemists who design fireworks are experts on many kinds of explosions. Many chemists who design fireworks also work on military applications such as producing brightly colored smoke for signaling purposes. Those who dispose of bombs may need to know how these kinds of explosions are made. 9. Pyrotechnic chemists are working to make fireworks safer for both people and the environment. It is important for pyrotechnic chemists to develop stable compounds that only explode/ignite in the sky. It is also important to make them as environmentally friendly as possible. 10.This article may change my views on fireworks. Opinions will vary.





















