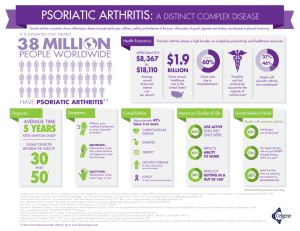
Measure Set Title/Number Type Denominator Numerator Psoriasis
advertisement
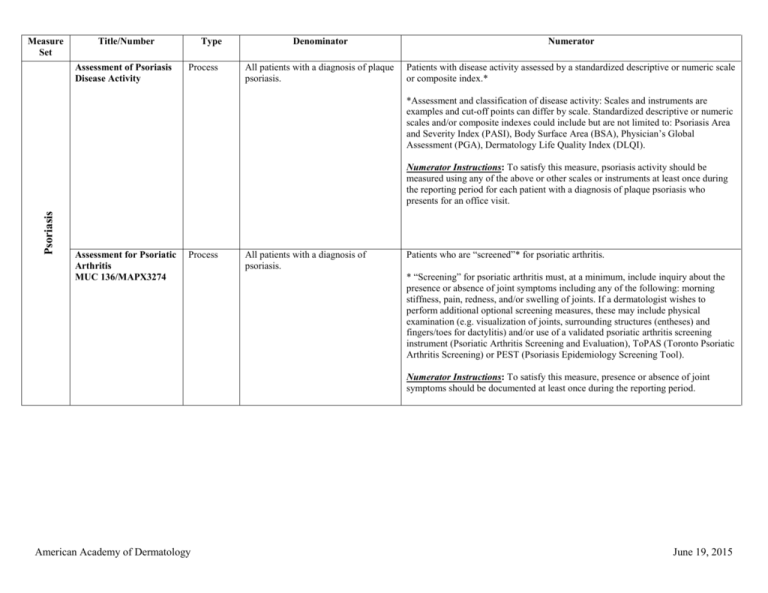
Measure Set Title/Number Assessment of Psoriasis Disease Activity Type Process Denominator Numerator All patients with a diagnosis of plaque psoriasis. Patients with disease activity assessed by a standardized descriptive or numeric scale or composite index.* *Assessment and classification of disease activity: Scales and instruments are examples and cut-off points can differ by scale. Standardized descriptive or numeric scales and/or composite indexes could include but are not limited to: Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), Physician’s Global Assessment (PGA), Dermatology Life Quality Index (DLQI). Psoriasis Numerator Instructions: To satisfy this measure, psoriasis activity should be measured using any of the above or other scales or instruments at least once during the reporting period for each patient with a diagnosis of plaque psoriasis who presents for an office visit. Assessment for Psoriatic Arthritis MUC 136/MAPX3274 Process All patients with a diagnosis of psoriasis. Patients who are “screened”* for psoriatic arthritis. * “Screening” for psoriatic arthritis must, at a minimum, include inquiry about the presence or absence of joint symptoms including any of the following: morning stiffness, pain, redness, and/or swelling of joints. If a dermatologist wishes to perform additional optional screening measures, these may include physical examination (e.g. visualization of joints, surrounding structures (entheses) and fingers/toes for dactylitis) and/or use of a validated psoriatic arthritis screening instrument (Psoriatic Arthritis Screening and Evaluation), ToPAS (Toronto Psoriatic Arthritis Screening) or PEST (Psoriasis Epidemiology Screening Tool). Numerator Instructions: To satisfy this measure, presence or absence of joint symptoms should be documented at least once during the reporting period. American Academy of Dermatology June 19, 2015 Tuberculosis Prevention for Psoriasis, Psoriatic Arthritis and Rheumatoid Arthritis Patients on a Biological Immune Response Modifier PQRS 337 Process All patients with a diagnosis of psoriasis, psoriatic arthritis, or rheumatoid arthritis who are on a biologic immune response modifier. Patients who have a documented negative annual TB screening or have documentation of the management of a positive TB screening test with no evidence of active tuberculosis, confirmed through use of radiographic imaging (i.e. chest xray, CT). Denominator Note: A patient would be considered denominator eligible for Measure #337 for reporting purposes, if the patient meets the denominator criteria with diagnosis of psoriasis or psoriatic arthritis or rheumatoid arthritis AND is on a biologic immune response modifier prescribed by the provider being evaluated for the measure. Clinical Response to Oral Systemic or Biologic Medications MUC 134/MAP X3726 Outcome All patients with a diagnosis of psoriasis and treated with an oral systemic or biologic medication for psoriasis for at least 6 months. Patients who have a documented physician global assessment (PGA; 6-point scale), body surface area (BSA), psoriasis area and severity index (PASI) and/or dermatology life quality index (DLQI) that meet any one of the below specified benchmarks. Numerator Instructions: To satisfy this measure, a patient must achieve any ONE of the following: a. PGA (6-point scale) ˂ 2 (clear to mild skin disease) b. BSA < 3% (mild disease) c. PASI < 3 (no or minimal disease) d. DLQI < 5 (no effect or small effect on patient’s quality of life). American Academy of Dermatology June 19, 2015