Chemistry 6 mark questions
advertisement

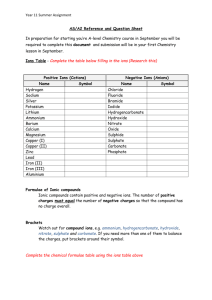
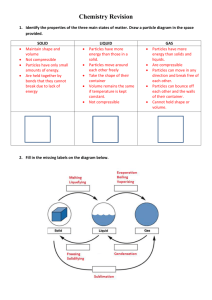
Chemistry Six Mark Question: C4 a: Describe Daltons atomic theory and how the work of J.J Thomson, Rutherford and Bohr contributed to the development of the theory of the atomic structure C4b: Describe the structure of Sodium Chloride in terms of ionic structure and how this explains the properties of it. C4c: Describe how the evidence an d observations of Dobereiner, Newlands and Mendeleev led to the development of the periodic table. C4d: Describe how to use the flame test to identify the presence of lithium , sodium and potassium compounds C4e: Explain in terms on terms of electron gain the trend in reactivity of the group 7 elements C4f: Describe the use of sodium hydroxide solution to identify the presence of transition metal ions in solution C4g: Describe what is meant by the term superconductor and its potential benefits C4h: Describe the water purification process and how you can further test ions C5a: When magnesium is heated it ends up as a white powder, the powder is heavier, you began with 48g of magnesium, and end with 80g of Magnesium oxide. Explain what has happened. C5b: Calculate the Empirical formula of a compound that contains 2.04%H, 32.65%S and 65.31% O. Calculate the relative formula mass of this compound. C5c : Explain how to perform a 1 in 10 dilution; include the equipment you would use and the volumes of liquids. C5d : You start off with 25cm3 of sodium hydroxide that has a concentration of 0.100 moles per dm3. It takes 49cm3 of hydrochloric acid to neutralise the sodium hydroxide. What is the concentration of the hydrochloric acid used? C5e : Sketch diagrams to show ways that can be used to collect and measure gases. Explain how each is used and how data is collected. C5f : 2SO2(g) + O2(g) 2SO3(g) The conditions for the contact process are: Vanadium Pentoxide catalyst, 450°C and atmospheric pressure. Explain why these conditions are chosen. C5g: Explain the difference between a strong and a weak acid, giving examples for each. C5h : Silver nitrate is made from silver ions and nitrate ions. Sodium sulphate is made from sodium ions and sulphate ions . The two solutions react to form a precipitate of silver sulphate. Write balanced ionic equations for the reactions forming silver nitrate and sodium sulphate. Write the symbol equation for the reaction between silver nitrate and sodium sulphate, with state symbols. C6a : Label the diagram; include half equations and explain what products are formed at the anode and cathode C6b : Describe a fuel cell and explain advantages and disadvantages of using it to generate electricity, include equations. C6c : Explain methods of protecting iron from rusting C6d : C6e : Explain how CFCs deplete the ozone layer, include the equations showing the production of the chlorine radical, destruction of the ozone and formation of chlorine gas to terminate the reaction. C6f : Describe the causes of temporary and permanent hardness and how hardness can be removed, include equations where appropriate. C6g: Explain Saponification of fats and oils, include a description of the reaction and a word equation. C6h : Explain, in terms of intermolecular forces, how a dry cleaning solvent removes stains. Draw diagrams to help you to explain.