NTeQ Lesson Plan Density
advertisement

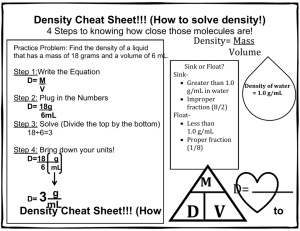
NTeQ Lesson Plan Introduction to Density: Grade 8 Learning Objectives Standards NETS Creativity and Innovation – Students demonstrate creative thinking, construct knowledge, and develop innovative products and processes using technology. o Use models and simulations to explore complex systems and issues. Gizmo Density simulation program Communication and Collaboration – Students use digital media and environments to communicate and work collaboratively, including at a distance, to support individual learning and contribute to the learning of others. o Contribute to project teams to produce original works or solve problems. Collaborative explanations to data Research and Information Fluency – Students apply digital tools to gather, evaluate and use information. o Process data and report results. Analyze data from simulation and use it to explain discover by inquiry the mathematical relationship between mass, volume and density. Critical Thinking – Students use critical thinking skills to plan and conduct research, manage projects, solve problems, and make informed decisions using appropriate digital tools and resources. o Collect and Analyze data to identify solutions and/or make informed decisions. Analyze data from the simulation and observations Digital Citizenship – Students understand human, cultural, and societal issues related to technology and practice legal and ethical behavior. o Exhibit a positive attitude toward using technology that supports collaboration, learning, and productivity. Work together to analyze data and apply concepts to explain real life situations Maryland State Curriculum 8.B.1.a Use appropriate tools to gather data and provide evidence that equal volumes of different substances usually have different masses. Standard 1 Skills and Processes: B.1 Review data from a simple experiment, summarize the data, and construct a logical argument about the cause-and-effect relationships in the experiment Specific Lesson Objectives Students will discover by inquiry the mathematical relationship between mass, volume and density. Students will apply the concept of density to explain why some objects float on others. Students will use the skills illustrated in the simulation to calculate the density of real objects in the lab and discover that large and small samples of the same substance have the same density. Students will use the density concepts learned to explain several real life examples of floating objects, like ships and hot air balloons. Computer Functions A computer simulation (Gizmos: www.explorelearning.com) will be used to have students discover the mathematical relationship between mass, volume and density. Students will view videos and read articles with graphics of real life objects such as the sinking of the Titanic and hot air balloons. Students will choose one topic and use presentation software (PowerPoint, Prezi or Padlet) to make a short presentation of how these are explained using the density concepts learned in class. Specify Problem How are mass, volume and density of a substance related? What causes an object to float? Why can a ship composed of steel float, when a piece of steel will sink in a bucket of water. What real life situations are explained by the concepts we have learned in this lesson? Research and Analysis Students will gather data using the Gizmos Density simulation lab. They will analyze the data in their lab groups in order to discover by inquiry the relationship between the density of an object and whether or not it floats. Students will view videos and animations of real life objects such as hot air balloons, The Titanic, submarines or student choice (approved by teacher) and apply the knowledge acquired in the activity to explain how they work. Students will need to have background knowledge of the definitions of mass and volume, and the fact that all matter is made of molecules. Results Presentation Groups will choose a real life object, such as a hot air balloon, the sinking of the Titanic, a submarine, or another approved object, and will work collaboratively to construct a short (3-5 minute) multimedia presentation. o o The presentation will use the concepts learned about density to explain how the object works or the incident happened. Students should include at least one picture, or graphic or video in their presentation. Planning Computer Activities Students will each work at their own computer to complete the simulation and collect the data. Students will rotate from individual computer use to group discussion to analyze the data and complete the Think Sheet. After the Think Sheet is complete, students will work in the actual science lab. Students will return to computers and investigate the topics individually. They will then return to their group to decide which topic they want to choose for their presentation. When the group has decided, they will divide up the research and each student will make a part of the presentation (a few slides). During Computer Use Students will complete the density simulation www.explorelearning.com Density Laboratory individually. They will complete the worksheet which will guide them through collecting the data. Students will return to the computers after completing the Think Sheet and actual lab activity to research various applications of the knowledge about density they have gained from this activity. o Students will be given a list of possible topics (why the Titanic sank, how hot air balloons, how submarines work) and a list of some web sites where they could begin their research. o They may choose their own topic of interest as long as it is approved by the teacher. o Websites (a list of these will be on the teacher’s www.delicious.com site under the tag of density. Students will be able to access these links there.) www.howstuffworks.com www.livescience.com/19599 www.eballoon.org www.PBSkids.orgdragonflytv/show/balloon www.discoveryeducation.com Students will return to their groups and decide which topic to choose. Students will work collaboratively to make a short multimedia presentation using their choice of presentation software (3-5 minutes) that shows how their topic is related to density. Before Computer Use A demonstration will be done where a piece of steel is dropped in a bucket of water. Students will be presented with the following question: Why can a boat made of steel float when a piece of steel in a bucket of water sinks? Students will be given a few minutes to discuss this with their groups and a short class discussion will be conducted. Students will then be told they will be able to answer this question after the activity is completed. After Computer Use Students will be moving back and forth from the computers. o After students complete the simulations on their own, they will move to their tables to complete the Think Sheet collaboratively, which will be checked by the teacher. o Then students will go to the actual lab and practice the skills learned in the simulation with real pieces of metal. o After spending some time researching the different topics, students will return to their groups to discuss and choose a topic for their presentation. Supporting Activities Students will investigate ways in which density is used in industry and science. An example might be explaining how it is used to separate crude oil into its usable parts or how it is used in the primary steps in sewage treatment. Assessment Formative assessment – Check the group’s answers to the Think Sheet to make sure the proper connections between mass, volume and density and floating have been discovered by the group. Summative assessment- A rubric will be designed to assess the multimedia presentation by each group. o Students must show how density concepts explain how their chosen topic works. o Students must have at least one picture, graphic or video in their presentation. The students will be given a short quiz where they have to briefly answer the question presented at the beginning of the lesson: Why can a boat made of steel float when a piece of steel in a bucket sinks? Name: ______________________________________ Date: ________________________ Student Exploration: Density Laboratory (To be completed during the simulation individually) Vocabulary: buoyancy, density, graduated cylinder, mass, matter, scale, volume Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. Of the objects below, circle the ones you think would float in water. 2. Why do some objects float, while others sink? ____________________________________ _________________________________________________________________________ _________________________________________________________________________ Gizmo Warm-up The Density Laboratory Gizmo™ allows you to measure a variety of objects, then drop them in water (or other liquid) to see if they sink or float. 1. An object’s mass is the amount of matter it contains. The mass of an object can be measured with a calibrated scale like the one shown in the Gizmo. Drag the first object onto the Scale. (This is object 1.) What is the mass of object 1? _______________________________ 2. An object’s volume is the amount of space it takes up. The volume of an irregular object can be measured by how much water it displaces in a graduated cylinder. Place object 1 into the Graduated Cylinder. What is the volume of object 1? _____________________________ Note: While milliliters (mL) are used to measure liquid volumes, the equivalent unit cubic centimeters (cm3) are used for solids. Therefore, write the volume of object 1 in cm3. 3. Drag object 1 into the Beaker of Liquid. Does it sink or float? ________________________ Activity A: Float or sink? Get the Gizmo ready: Drag object 1 back to the shelf. Check that Liquid Density is set to 1.0 g/mL. Question: How can you predict whether an object will float or sink? 1. Observe: Experiment with the different objects in the Gizmo. Try to determine what the floating objects have in common and what the sinking objects have in common. 2. Form hypothesis: Compare the floating objects, then do the same for the sinking objects. A. What do the floating objects have in common? ______________________________ ___________________________________________________________________ B. What do the sinking objects have in common? ______________________________ ___________________________________________________________________ 3. Collect data: Measure the mass and volume of objects 1 through 12, and record whether they float or sink in the table below. Leave the last column blank for now. Object 1 2 3 4 5 6 7 8 9 10 11 12 Mass (g) Volume (cm3) Float or sink? Think Sheet (to be completed in your lab groups) 4. Analyze: Look carefully for patterns in your data. A. Does mass alone determine whether an object will float or sink? ________________ Explain: ____________________________________________________________ B. Does volume alone determine whether an object will float or sink? ______________ Explain: ____________________________________________________________ C. Compare the mass and volume of each object. What is true of the mass and volume of all the floating objects? ______________________________________________ D. What is true of the mass and volume of all the sinking objects? _________________ ___________________________________________________________________ 5. Calculate: The density of an object is its mass per unit of volume. Dense objects feel very heavy for their size, while objects with low density feel very light for their size. To calculate an object’s density, divide its mass by its volume. If mass is measured in grams and volume in cubic centimeters, the unit of density is grams per cubic centimeter (g/cm 3). Calculate the density of each object, and record the answers in the last column of your data table. Label this column “Density (g/cm3).” 6. Analyze: Compare the density of each object to the density of the liquid, 1.0 g/mL. This is the density of water. A. What do you notice about the density of the floating objects? ___________________ ___________________________________________________________________ B. What do you notice about the density of the sinking objects? ___________________ ___________________________________________________________________ 7. Draw conclusions: If you know the mass and volume of an object, how can you predict whether it will float or sink in water? _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Calculating Density to Identify Unknown Substances Metal Short Gold 2 Black Stripes Red Stripe Tall Silver Short gray Medium gray Blue stripe Large wooden block Mass g Volume mL Density g/mL end -start _______ ------ = end -start _______ ------- = end -start ________ ------- = end -start _______ ------- = end -start _______ ------- = end -start _______ ------- = end -start _______ ------- = L x W xH= ------- = ----x-----x-----= __________ Identification (use the chart provided) Large metal block L x W xH= ------- = ----x-----x-----= __________ Wood block small L x W xH= ------- = ----x-----x-----= __________ Small metal block L x W xH= ------- = ----x-----x-----= __________ Yellow liquid ------- = Clear liquid ------- =