Stoichiometry study guide
advertisement
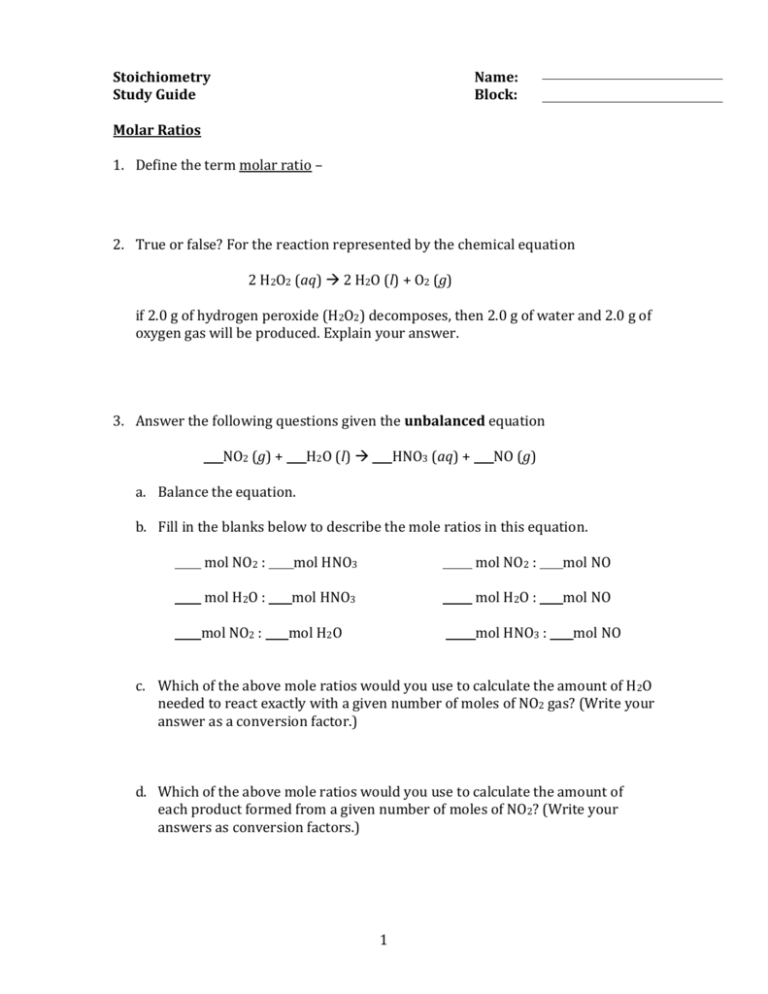
Stoichiometry Study Guide Name: Block: Molar Ratios 1. Define the term molar ratio – 2. True or false? For the reaction represented by the chemical equation 2 H2O2 (aq) 2 H2O (l) + O2 (g) if 2.0 g of hydrogen peroxide (H2O2) decomposes, then 2.0 g of water and 2.0 g of oxygen gas will be produced. Explain your answer. 3. Answer the following questions given the unbalanced equation NO2 (g) + H2O (l) HNO3 (aq) + NO (g) a. Balance the equation. b. Fill in the blanks below to describe the mole ratios in this equation. mol NO2 : mol HNO3 mol NO2 : mol NO mol H2O : mol HNO3 mol H2O : mol NO mol NO2 : mol H2O mol HNO3 : mol NO c. Which of the above mole ratios would you use to calculate the amount of H2O needed to react exactly with a given number of moles of NO2 gas? (Write your answer as a conversion factor.) d. Which of the above mole ratios would you use to calculate the amount of each product formed from a given number of moles of NO2? (Write your answers as conversion factors.) 1 e. Calculate the number of moles of each product that are formed from the complete reaction of 0.5 mol of NO2. f. Calculate how many moles of HNO3 are produced if 0.125 mol of NO forms. Mass Calculations 1. Define the term stoichiometry – 2. Describe how to find the molar mass of any compound. 3. Calculate the number of moles contained in each of the following samples: a. 2.01 g of silver b. 5.23 kg of sulfur dioxide c. 12.7 mg of iron (II) sulfate 4. Calculate the mass in grams of each of the following samples: a. 0.0219 mol of hydrogen gas b. 4.01 mol of potassium hydroxide 2 c. 7.21 x 10-3 mol of carbon dioxide 5. Draw a diagram (using general terms, not numbers) showing how to calculate the mass of reactant A if given 5.00g of reactant B. Reactant A + Reactant B Product C 6. Answer the following questions given the unbalanced equation NaHSO3 (aq) + NaOH (aq) Na2SO3 (aq) + H2O (l) a. Balance the equation. b. How many moles of NaOH are required to react completely with 25.0 g of NaHSO3? c. What mass of NaOH is required to react completely with 25.0 g of NaHSO3? d. What mass of Na2SO3 is produced from 25.0 g of NaHSO3? 3 e. What mass of NaOH is required to produce 15.0 g of H2O (assuming excess NaHSO3)? 7. Both propane (C3H8) and butane (C4H10) react with oxygen gas to form carbon dioxide and water. a. Write 2 balanced equations describing these reactions. b. If you have 10.0 g of C3H8 and C4H10, which will require a greater mass of oxygen for a complete reaction? Limiting Reactants and Percent Yield 1. Define the following terms: a. limiting reactant – b. theoretical yield – c. percent yield – 2. True or false: the limiting reactant can be identified as the reactant with the lowest number of moles. Explain your answer. 3. Why do many reactions fall short of producing the theoretical yield? 4 4. Answer the following questions given the unbalanced equation Li (s) + N2 (g) Li3N (s) a. Balance the equation. What type of reaction is this? b. If 56.0 grams of lithium are mixed with 56.0 grams of nitrogen gas, calculate the number of moles of each reactant that are present. c. Which is the limiting reactant? Which reactant is in excess? d. Calculate the mass of lithium nitride that will be produced from this reaction. e. How many grams of the excess reactant remain? f. If 6.5 x 104 g of N2 gas is reacted with excess lithium, what is the theoretical yield of lithium nitride? g. How much lithium nitride will be produced if the percent yield of the reaction is only 65%? 5