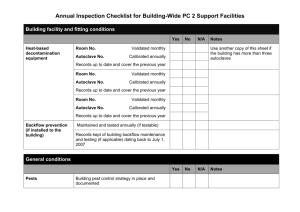
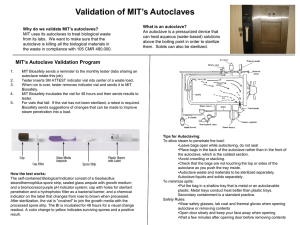
SOP(PRA) Waste Autoclave use
advertisement

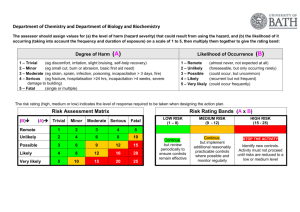
2013/2014 FACULTY OF COMPUTING, ENGINEERING & SCIENCES – SCHOOL OF SCIENCES RISK ASSESSMENT FORM Procedure: Academics complete Risk Assessment for all practical classes/activities, Technical team for all support aspects and forward to Faculty Health & Safety Advisor, this is then reviewed on an annual basis Experimenters complete Risk Assessment in consultation with project advisor and technical staff as appropriate No laboratory work is to commence without a suitable and sufficient risk assessment being signed off by the Faculty Health & Safety advisor or for experimenters by the project advisor and Faculty Health & Safety Advisor/nominated individual Experimenters to keep copies of Risk Assessments when working in the laboratories Notes: The risk assessment must be reviewed when any changes are made to the equipment, materials, procedure or personnel Technical staff can stop experimental work if no risk assessment is in place, or if, in their opinion, there is a risk to safety □√ Biology □ Forensics & Crime Science □ Geography Student: Name & email address: Ref No: MB22 Project advisor: Staff: IAN HOPKINS Ethical consideration: No ethics declaration √ Fast track Full ethics Date submitted/passed Title of project/module (include module number): Use and maintenance of Priorclave top-loading autoclave designated for waste treatment in R215 Autoclave Room Science Centre Description of experimental procedure/practical session This autoclave is designated to be used to sterilise laboratory waste prior to its final disposal as skip waste, and also clinical waste prior to its collection by a specialist disposal company. At present the autoclave used for this purpose is a 150 litre capacity Priorclave autoclave (*Serial No. 9374 – purchased in 1992). This unit is situated in R215 and is connected to the common series of vent piping shared with the other 3 autoclaves that serves to convey any contaminated steam and condensate outside the building. The range of solid and liquid wastes that are treated by autoclaving include cultures and media, blood, animal tissue, contaminated glassware, pipettes, contaminated clothing, sharps in autoclavable bins, other disposable and reusable articles, gloves, laboratory coats, paper towels and tissues. For processing, Sterilin (or equivalent brand) steam-sterilizable plastic bags containing waste or contaminated clothing are placed in a leakproof discard container inside the autoclave, with sterilisation indicator strips (Propper OK indicator strips, or equivalent brand) being placed inside each load. Bags or containers of clinical waste are labelled with 2-part indicator tags (Whitney Products Inc.) prior to autoclaving. Bags should be left OPEN with the top of the bag rolled outwards exposing the load to the steam generated within the pressure vessel .Contaminated glassware should be contained within autoclavable baskets. Care should be taken to ensure when loading the baskets or containers that they are not packed so tightly that steam can not penetrate the load properly. Once the autoclave is loaded, the pressure door can now be lowered and secured, and the safety cover closed. Settings for the autoclaving of waste should be 121º C at a pressure of 1 Bar for a minimum of 30 minutes. Under normal circumstances, this autoclave cannot be opened until the temperature on the external dial falls below 40º C (or lower). Once the autoclave is open, the contents of the bags of processed agar waste should be allowed to solidify after which they can be treated as normal refuse (placed in bin liners) and removed to the general waste skip located outside the building. The removable part of the indicator tag attached to each clinical waste bag can be torn off and entered into the Infectious Waste Control Logbook providing a permanent record of the treatment. Once cool, clinical waste bags are placed in larger designated red/yellow plastic bags and removed to a special skip exterior to the Science Centre from where it is collected by a designated specialist disposal company at regular intervals. Treated sharps containers are also kept in the same skip prior to collection. Any processed glassware should be washed out with large volumes of water. Routine maintenance involves a visual check for damage; changing the water in the vessel and cleaning weekly or as required (dependent on use); lubricating the gasket and locking arms monthly; and checking the safety valve and drainage. All this should be done following the directions given in the autoclave instruction handbook. Records of servicing/repair carried out by Priorclave engineers are to be kept by the Safety Officer, whilst records of Insurance inspections are kept in R236. There is no reasonable alternative to sterilising by autoclave that is less hazardous. Hazards inherent in the work, record details and possibility of risk/harm: (Equipment, procedures, invertebrate work, body fluid sampling etc.) Record precautions which will be taken: (e.g. Any standard operating procedures to follow, SAF codes, faculty policies) The autoclave operates under high temperature and pressure. It is designed to prevent access whist there is any danger of exposure to high temperature or release of pressure. The autoclave will not commence an operating cycle if not The autoclave sterilises its contents by raising both temperature and pressure, and generating steam. Thermal gloves should always be worn for opening the autoclave (unless it is known with certainty that contents 2 properly sealed, or low on water, for example. Operational malfunction due to a failure of the electrical supply. Or due to a fault within the autoclave may result in the load inside being only partially sterilised or completely unsterilized. Autoclaving of agar presents particular hazards i.e. possibility of scalding or burning. COSSH assessment (harmful substances) Hazardous to the Aquatic Environment (W) Acute Toxicity (T+) Gases Under Pressure (G) will be at room temperature i.e. the unit has been allowed to cool fully overnight). The vessel is tested annually by an insurance engineer, records of such testing are kept in R236. The autoclave is only to be operated by trained staff, and should be kept well-maintained following the manufacturers guidelines. Operational instructions are stored in R215. When loading the autoclave with waste, ample room must be allowed for steam to penetrate through the load or else full sterilisation will not be achieved. Indicator strips or tags should be inspected following the completion of a sterilisation cycle to ensure that requisite colour changes have occurred; failure of a these to exhibit the expected colour change would suggest that the load has not been fully sterilised and that it must be re-autoclaved. Minimum handling precautions Fume cupboard (F) Safety glasses (SG) Microbiological cabinet (Cab) Laminar flow cabinets (LF) Gloves (GL) Face mask (M) Respirator (R) Other All INFORMATION CAN BE FOUND WITHIN MSDS (MATERIAL SAFETY DATA SHEETS) ON THE INTERNET, SCIENCES CHEMICAL DATABASE ON-LINE OR WITHIN EACH OF THE LABORATORIES Corrosive (C) Caution (H, I) Explosive (E) Oxidising (O) Flammable (F) Longer Term Health Hazards (M) 3 Chemicals involved (including Products): COSHH information as above: Minimum handling precautions: Microorganisms: Classification: Minimum handling precautions: Various ACDP Hazard Group 1&2 Observation of university codes for good laboratory practice (SAF001) and disposal of contaminated waste (SAF003) Other Materials: Hazards: Minimum handling precautions: Quantity to be used: GL, SG various Observation of university codes for good laboratory practice (SAF001) and disposal of contaminated waste (SAF003) various Agars (if molten)/broths Clinical waste biohazard Contaminated laboratory waste biohazard Vacuum grease (for periodic treatment of autoclave gaskets) Do any of the above substances have a workplace exposure limit (WEL) please state value and precautions: Quantity to be used: ml/g/% solution/M various 1-2 mls Yes No √ Disposal information (How will all reactants/products be disposed of?) Any sterilised molten agar waste or broth held in glass bottles should be poured off down the drain of the main laboratory sink accompanied by flushing with a large volume of water. Bottles can then be washed out and reused. Sterilised solidified agar waste allowed to cool following autoclaving, can then be treated as regular waste and taken to a skip for disposal as landfill. Treated clinical waste and treated sharps bins once cool, are taken and stored in a designated yellow skip for separate collection by a designated specialist disposal company. Have you checked all materials used are not hazardous to the environment? YES 4 Any special conditions specified as part of the permission to carry out the work/procedure and actions needed to minimise risk e.g. adherence to HTA or body fluid policy 1.52, completion of fieldwork risk assessment etc. University codes for the disposal of contaminated waste (SAF003) to be observed at all times. Prerequisites for use of the autoclave are that the unit should have been subject to regular servicing by Priorclave engineers, routine maintenance specified by the manufacturer carried by trained technical staff (all biological sciences technical staff are trained in autoclave use), and that the unit been cleared for future use by an insurance assessment. The unit should only be operated by technical staff. If during its operation the autoclave demonstrates any kind of fault that cannot readily be rectified by the technical staff (i.e. a low water fault indication simply requires topping up of the internal reservoir in the autoclave), or else behaves at all abnormally, then its use must immediately cease and the matter quickly referred to a Priorclave engineer. Project advisor/Academic comments: (Any disability issues to be aware of?) n/a Staff/Project advisor- What level of risk do you assign with this work? High Medium Low √ Date: July 22nd 2013 Faculty H&S approval Date of Review August 2015 Audra Jones Date: 29th August 2014 Any other comments? 5