histo 755 to 762 [2-9
advertisement

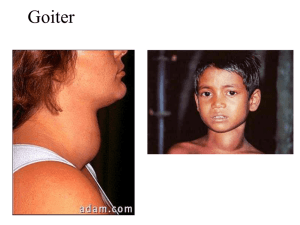
histo 755 to 762 Thyroid Gland Located in anterior neck region adjacent to larynx and trachea; consists of 2 lateral lobes connected by isthmus o Pyramidal lobe can extend upward from the isthmus (40% of population) o Thin CT capsule surrounds the gland; sends trabeculae into parenchyma that partially outline irregular lobes and lobules Thyroid follicles – functional units of thyroid Begins to develop during 4th week of gestation from primordium originating as endodermal thickening of floor of primitive pharynx; primordium grows caudally and forms duct-like invagination (thyroglossal duct) o Thyroglossal duct descends through tissue to in front of the trachea, where it divides into 2 lobes o During downward migration, thyroglossal duct undergoes atrophy (pyramidal lobe is remnant of this) o At 9th week of gestation, endodermal cells differentiate into plates of follicular cells that become arranged into follicles o By week 14, well-developed follicles lined by follicular cells contain colloid in lumen o During week 7, epithelial cells lining invagination of 4th branchial pouches (ultimobranchial bodies) start migration toward developing thyroid gland and become incorporated into lateral lobes After fusing with thyroid, ultimobranchial body cells disperse among follicles giving rise to parafollicular cells that become incorporated into follicular epithelium Thyroid follicle – roughly spherical cyst-like compartment with wall formed by simple cuboidal or low columnar epithelium (follicular epithelium) o Hundreds of thousands of follicles that vary from 0.2-1.0 mm in diameter constitute nearly entire mass o Follicles contain gel-like mass called colloid; apical surfaces of follicular cells in contact with colloid, and basal surfaces rest on typical basal lamina Parenchyma of thyroid gland composed of o Follicular cells (principal cells) – responsible for production of T4 and T3; vary in shape and size Slightly basophilic basal cytoplasm with spherical nuclei containing one or more prominent nucleoli; Golgi apparatus has supranuclear position Lipid droplets and PAS-positive droplets Organelles commonly associated with both secretory and absorptive cells (typical junctional complexes at apical end and short microvilli on apical cell surface) Numerous profiles of rER present in basal region Small vesicles present in apical cytoplasm Abundant endocytotic vesicles (colloidal resorption droplets) and lysosomes present in apical cytoplasm o Parafollicular cells (C cells) – located in periphery of follicular epithelium and lie within the follicle basal lamina; have no exposure to follicle lumen; secrete calcitonin (regulates calcium metabolism) Pale staining and occur as solitary cells or in small clusters of cells At electron microscope level, they have numerous small secretory vesicles and prominent Golgi o Extensive network of fenestrated capillaries derived from superior and inferior thyroid arteries surrounds follicles o Blind-ended lymphatic capillaries present in interfollicular CT and provide second route for conveying hormones from gland Thyroxine (tetraiodothyronine or T4) and triiodothyronine (T3) synthesized and secreted by follicular cells; regulate cell and tissue basal metabolism and heat production and influence body growth and development o Secretion regulated by TSH released from anterior lobe of pituitary gland Calcitonin (thyrocalcitionin) – synthesized by parafollicular cells and is physiologic antagonist to PTH o Lowers blood Ca2+ by suppressing resorptive action of osteoclasts and promotes Ca2+ deposition in bones by increasing rate of osteoid calcification o Secretion regulated directly by blood Ca2+; high levels of Ca2+ stimulate secretion, and low levels inhibit secretion; secretion of calcitonin unaffected by hypothalamus and pituitary gland o Calcitonin can be secreted by several endocrine tumors (e.g., medullary carcinoma of thyroid) o Can be used to treat disorders associated with excess bone resorption (e.g., osteoporosis and Paget’s disease), but no clinical disease associated with its deficiency or absence after total thyroidectomy Principal component of colloid is iodinated glycoprotein (thyroglobulin); colloid also contains several enzymes and other glycoproteins; stains with both basic and acidic dyes o Thyroglobulin is inactive storage form of thyroid hormones; active thyroid hormones liberated from thyroglobulin and released into fenestrated blood capillaries that surround follicles after processing Synthesis of thyroid hormones takes place in thyroid follicle o Precursor of thyroglobulin synthesized in rER of follicular epithelial cells; thyroglobulin posttranslationally glycosylated in rER and Golgi apparatus before being packaged into vesicles and secreted by exocytosis into lumen of follicle o Follicular epithelial cells actively transport iodide from blood into cytoplasm using ATPase-dependent sodium/iodide symporters (NIS) NIS mediates active iodide uptake in basolateral membrane of follicular epithelial cells Iodide ions then diffuse toward apical membrane, where they are transported to lumen of follicle by iodide/chloride transporter (pendrin) located in apical cell membrane Iodide then immediately oxidized to iodine (active form); catalyzed by membrane-bound thyroid peroxidase (TPO) o Iodine atoms added to specific tyrosin residues of thyroglobulin (1-2 depending on which hormone it will become); occurs in colloid at microvillar surface of follicular cells and catalyzed by TPO Addition of one iodine makes monoiodotyrosine (MIT), and addition of 2 makes diiodotyrosine (DIT) residue o Formation of T3 and T4 by oxidative coupling reactions of 2 iodinated tyrosine residues in close proximity (DIT + MIT = T3, DIT + DIT = T4) After iodination, T4 and T3, as well as DIT and MIT residues still linked to thyroglobulin molecule, stored as colloid in lumen of follicle o In response to TSH, follicular cells take up thyroglobulin from colloid by receptor-mediated endocytosis Lysosomal pathway – thyroglobulin internalized and transported in endocytotic vesicles to early endosomes; eventually mature into lysosomes or fuse with existing lysosomes Resorption of thyroglobulin confirmed by presence of large endocytotic vesicles (colloidal resorption droplets) in apical region of follicular cells Thyroglobulin degraded by lysosomal proteases into constituent amino acids and carbs, leaving free T4, T3, DIT, and MIT molecules Under physiological conditions, this is major pathway of colloid resorption Transepithelial pathway – thyroglobulin transported intact from apical to basolateral surface of follicular cells; to enter this pathway, thyroglobulin binds to receptor megalin (transmembrane protein expressed at apical surface of follicular cell directly facing colloid Thyroglobulin internalized by megalin avoids lysosomal pathway and endocytic vesicles delivered to basolateral membrane of follicular cells In pathologic conditions of high TSH or TSH-like stimulation, megalin expression increased and large amounts of thyroglobulin follow transepithelial pathway Reduces extent of T4 and T3 release by diverting thyroglobulin away from lysosomal pathway Patients with hyperthyroidism have detectable amounts of circulating thyroglobulin that contains portion of megalin receptor If levels of TSH remain high, amount of colloid in follicle reduced because it is synthesized, secreted, iodinated, and resorbed too rapidly to accumulate o Majority of T4 and T3 liberated from thyroglobulin in lysosomal pathway in T4 to T3 ratio 20:1 Cross basal membrane and enter blood and lymphatic capillaries Most of released hormone immediately bound to either thyroxin-binding protein (70%) or to nonspecific prealbumin fraction of serum protein (25%), leaving ~5% free circulating hormones Very small amounts of T4 to T3 released bound to thyroglobulin Only follicular cells capable of producing T4, whereas most T3 (5x more active than T4) produced through conversion from T4 by kidneys, liver, and heart Free circulating hormones function in feedback system that regulates secretory activity of thyroid Once uncoupled from thyroglobulin, DIT and MIT molecules deiodinated within cytoplasm of follicular cells to release tyrosine and iodide, which are recycled Thyroid hormones essential to normal growth and development o In normal pregnancy, both T3 and T4 cross placental barrier and are critical in early stages of brain development o Fetal thyroid gland begins to function during 14th week of gestation and contributes additional thyroid hormones o Thyroid hormone deficiency during fetal development results in irreversible damage to CNS, causing reduced numbers of neurons, defective myelination, and mental retardation o If maternal deficiency present before development of fetal thyroid gland, mental retardation is severe o Thyroid hormones also stimulate gene expression for GH in somatotropes; without thyroid hormones, generalized stunted body growth typical o Combination of mental retardation and stunted body growth is congenital hypothyroidism Abnormal Thyroid Function Most common symptom of thyroid disease is goiter (enlargement of thyroid gland); may indicate either hyperthyroidism or hypothyroidism Hypothyroidism – can be caused by insufficient dietary iodine (iodine-deficiency goiter, endemic goiter) or by autoimmune diseases such as autoimmune thyroiditis (Hashimoto’s thyroiditis) o Autoimmune thyroiditis characterized by presence of abnormal autoimmunoglobulins directed against thyroglobulin (TgAb), thyroid peroxidase (TPOAb), and TSH receptor (TSHAb) Results are thyroid cell apoptosis and follicular destruction o Low levels of circulating thyroid hormone stimulate release of excessive amounts of TSH, which cause hypertrophy of thyroid through synthesis of more thyroglobulin o Adult hypothyroidism (formerly called myxedema) characterized by mental and physical sluggishness Edema occurs in severe stages of hypothyroidism and is caused by accumulation of large amounts of hyaluronan in ECM of CT of dermis Hyperthyroidism (toxic goiter, Graves’ disease) – excessive amounts of thyroid hormones released into circulation o Individuals with Graves’ disease have detectable levels of autoantibodies that bind to TSH receptors on follicular cells and stimulate adenylate cyclase activity, resulting in increased cAMP in follicular cells leading to continuous stimulation of cells and increased thyroid hormone secretion Because of negative feedback, levels of TSH in circulation usually normal o Thyroid gland undergoes hypertrophy, and thyroid hormone secreted at abnormally high rates, causing increased metabolism o Increased sympathetic nerve activities with increased metabolic rate (weight loss, excessive sweating, tachycardia, and nervousness) o Noticeable features include exophthalmos from increased sympathetic activity and increased deposition of ECM in adipose tissue located behind eyeball o Thyroid gland enlarged (goiter) o Microscopic features include presence of columnar follicular cells Because of high utilization of colloid, follicle tends to be depleted in areas of contact with apical surface of follicular cells o Treatment is either surgical removal of thyroid gland or radiotherapy by ingestion of radioactive iodine, which destroys most active follicular cells Parathyroid Glands Small endocrine glands closely associated with thyroid; superior and inferior parathyroid glands (2 pairs) Usually located in CT on posterior surface of lateral lobes of thyroid gland; number and location may vary Surrounded by thin CT capsule that separates it from thyroid; septa extend from capsule into gland to divide it into poorly defined lobules and separate densely packed cords of cells o CT more evident in adult, with development of fat cells that increase with age and ultimately constitute as much as 60-70% of glandular mass Receive blood supply from inferior thyroid arteries or anastomoses between superior and inferior thyroid arteries; rich networks of fenestrated blood capillaries and lymphatic capillaries surround parenchyma of parathyroids Embryologically, inferior parathyroid glands (and thymus) derived from 3rd branchial pouch, and superior glands come from 4th branchial pouch o Normally inferior parathyroids separate from thymus and come to lie below superior parathyroids; failure to separate results in atypical association of parathyroids with thymus in adult o Principal (chief) cells differentiate during embryonic development and are functionally active in regulating fetal Ca2+ metabolism o Oxyphil cells differentiate later in puberty Principal (chief) cells – more numerous parenchymal cells; responsible for regulating synthesis, storage, and secretion of PTH o Small, polygonal cells with centrally located nucleus; pale-staining, slightly acidophilic o Contains lipofuscin-containing vesicles, large accumulations of glycogen, and lipid droplets o Small, dense, membrane-limited vesicles seen with TEM (storage of PTH) o Can replicated when chronically stimulated by changes in blood Ca2+ Oxyphil cells – constitute minor portion of parenchymal cells and not known to have secretory role; found singly or in clusters; cells more rounded and larger than principal cells o Have distinctly acidophilic cytoplasm o Mitochondria (often with bizarre shapes and sizes) almost fill cytoplasm and are responsible for strong acidophilia o No secretory vesicles and little if any rER o Cytoplasmic inclusion bodies consist of occasional lysosomes, lipid droplets, and glycogen distributed among mitochondria PTH essential for life; if glands totally removed, death will ensue because muscles (including laryngeal and other respiratory muscles) go into tetanic contraction as blood Ca2+ falls o PTH binds to specific PTH receptor on target cells that interacts with G protein to activate secondmessenger system o PTH release causes level of Ca2+ in blood to increase; also reduces concentration of serum PO4 o Secretion of PTH regulated by serum Ca2+ through simple feedback system (parathyroid calcium-sensing receptors on principal cells) PTH functions on o Bone tissue – receptors for PTH on osteoprogenitor cells, osteoblasts, osteocytes, and bone lining cells Osteoclasts don’t have PTH receptors (indirectly activated by RANK-RANKL signaling mechanism of osteoblasts Binding of PTH to receptors on osteoblasts increases local RANK production and decreases osteoprogerin (OPG) secretion; stimulates osteoclast differentiation, which leads to increased bone resorption and release of calcium and phosphates into extracellular fluid PTH has anabolic effect on bone that leads to increased bone mass, so used in treatment of osteoporosis o Kidney excretion of calcium decreased by PTH stimulation of tubular reabsorption, conserving Ca2+ o Urinary phosphate excretion increased by PTH secretion, lowering phosphate concentration in blood and extracellular fluids o Kidney conversion of 25-OH vitamin D3 to hormonally active 1,25-(OH)2 vitamin D3 regulated primarily by PTH, which stimulates activity of 1α-hydroxylase and increases production of active hormone o Intestinal absorption of calcium increased under influence of PTH Vitamin D3 has greater effect than PTH on intestinal absorption of calcium Peak increase after release of PTH not reached for several hours; long-term homeostatic action Calcitonin rapidly lowers blood Ca2+ and has peak effect after about 1 hour; acute homeostatic action