Lesson 4 - Bonding Questions ANSWERS
advertisement

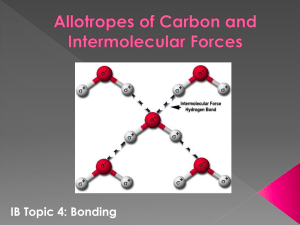
SBI4U Date:__________________ Bonding Questions - ANSWERS 1. What determines the strength of a chemical bond? a. Determined by the ‘bond order’. In other words, the number of electrons shared between atoms. The more electrons shared, the stronger the bond 2. What physical properties would be used to measure the strength of intermolecular bonding? a. State of matter. Gases have least bond strength, solids the greatest. Also, boiling point. A lower BP means less energy required to break apart the bonds. 3. Which of the following has a higher boiling point? Why? a. H2O or H2Se i. H2O. Water has stronger intramolecular bonds as O has a higher EN b. CH4 or CH3Cl i. CH3Cl. CH3Cl is a polar molecule, CH4 is non-polar. Polar molecules always have a higher boiling point as their intermolecular forces are stronger ie. Dipoledipole or hydrogen bond. 4. Predict which of the following substances will have the highest boiling point and why: methane CH4, ethane C2H6 or propane C3H8. a. C3H8 will have the highest. It’s the largest and heaviest molecule therefore requires the most energy to break apart the bonds. None are polar. 5. A) Determine the bond polarity of N-H in an ammonia molecule NH3 a. NH3 is a polar molecule. The N is partially negatives and the H’s will be partially positive B) What type of interactions will occur between water and ammonia? a. Water and ammonia are both polar therefore the ammonia will dissolve in water. The negative ends of the ammonia molecule will orient themselves to the positive O atoms of the water molecule. 6. Explain why intermolecular bonding between polar molecules is stronger than intermolecular bonding between non-polar molecules. a. Non-polar molecules have equal distribution of charges so the primary force of attraction is London Dispersion, the weakest type of attraction. 7. Explain why some covalent substances dissolve in water, while other covalent substances do not? a. Any covalent molecule that is non-polar will not dissolve in water. The highly polar ends of the water molecule will not be able to pull apart the non-polar solute. 8. Do water and oil mix. Explain why. No. Oil is non-polar. 9. What effect do the polarity, size and shape of a molecule have on the physical properties of the molecule? a. Typically the more polar a molecule is, the larger and more asymmetrical it is will result in a larger amount of energy needed to break apart the bonds meaning it will have a higher boiling point, and will be liquid instead of gas etc. 10. Two molecules (Molecule A and Molecule B) have the same number of electrons and are similar in size. Give one possible explanation of why molecule A might have a higher boiling point than molecule B. a. The types of atoms that are bonded could have a larger difference in electronegativity accounting for the higher boiling point. 11. What happens to salt when mixed with water? Explain by discussing electronegativity and polarity. Draw a molecular diagram of how water and salt interact in solution. a. Salt is an ionic compound composed of positive Na ions and negative Cl ions. The positive H ends of the water molecule are highly attracted to the negative Cl ions. As well, the negative O end of the water molecule is attracted to the positive Na ions. This attraction pulls apart the NaCl and causes the ions to ‘mix’ in with the water molecules.