Name: Item Analysis of Unit 1 Exam Example Problems from Test
advertisement
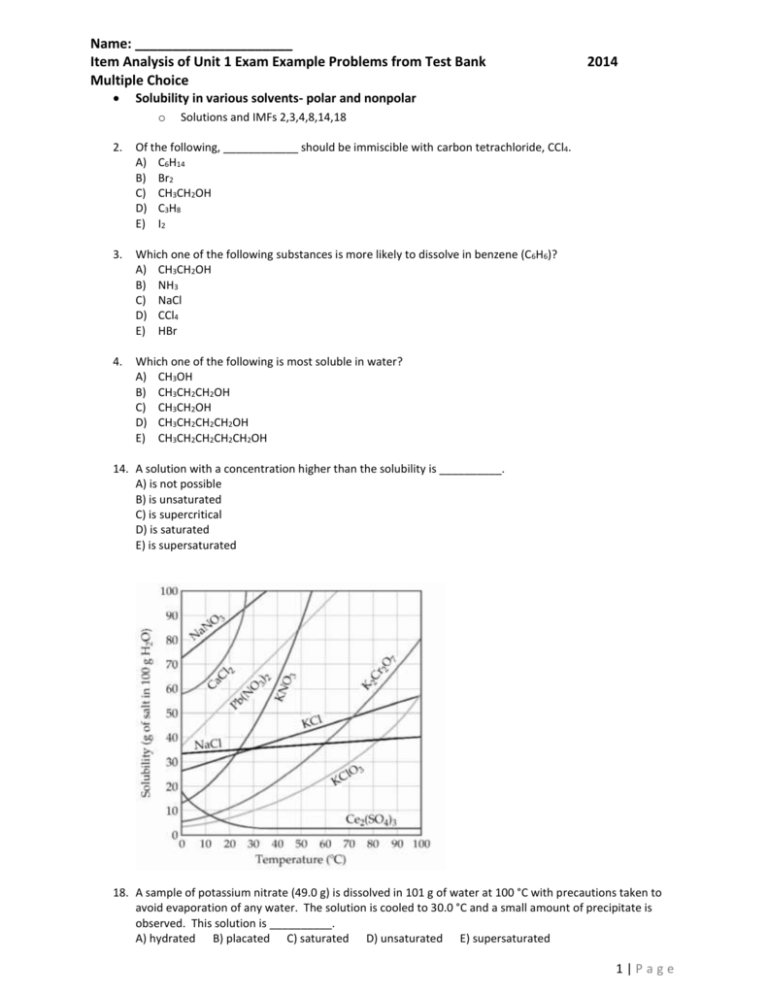
Name: _____________________ Item Analysis of Unit 1 Exam Example Problems from Test Bank Multiple Choice 2014 Solubility in various solvents- polar and nonpolar o Solutions and IMFs 2,3,4,8,14,18 2. Of the following, ____________ should be immiscible with carbon tetrachloride, CCl4. A) C6H14 B) Br2 C) CH3CH2OH D) C3H8 E) I2 3. Which one of the following substances is more likely to dissolve in benzene (C6H6)? A) CH3CH2OH B) NH3 C) NaCl D) CCl4 E) HBr 4. Which one of the following is most soluble in water? A) CH3OH B) CH3CH2CH2OH C) CH3CH2OH D) CH3CH2CH2CH2OH E) CH3CH2CH2CH2CH2OH 14. A solution with a concentration higher than the solubility is __________. A) is not possible B) is unsaturated C) is supercritical D) is saturated E) is supersaturated 18. A sample of potassium nitrate (49.0 g) is dissolved in 101 g of water at 100 °C with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and a small amount of precipitate is observed. This solution is __________. A) hydrated B) placated C) saturated D) unsaturated E) supersaturated 1|Page Mole fraction 31. A solution is prepared by dissolving 17.0 g of NH3 in 360.0 g of water. The mole fraction of NH3in the solution is __________. A) 0.064 B) 0.05 C) 0.940 D) 0.92 E) 16.8 32. The mole fraction of He in a gaseous solution prepared from 4.0 g of He, 20 g of Ar, and 10.0 g of Ne is __________. A) 0.50 B) 1.5 C) 0.20 D) 0.11 E) 0.86 33. The mole fraction of urea (MW = 60.0 g/mol) in a solution prepared by dissolving 15 g of urea in 40.5 g of H 2O is __________. A) 0.58 B) 0.37 C) 0.13 D) 0.10 E) 9.1 34. Calculate the mole fraction of HCl in a 10.0% (by mass) aqueous solution. A) 0.00111 B) 0.0344 C) 0.0520 D) 0.0548 E) 0.122 Molarity calculations o Solutions and IMFs 38-49 38. Which one of the following is not true concerning 2.00 L of 0.100 M solution of Ca3(PO4)2? A) This solution contains 0.200 mol of Ca 3(PO4)2. B) This solution contains 0.800 mol of oxygen atoms. C) 1.00 L of this solution is required to furnish 0.300 mol of Ca2+ ions. D) There are 6.02 x 1022phosphorus atoms in 500.0 mL of this solution. E) This solution contains 6.67 x 10-2mol of Ca2+. 39. When 0.500 mol of HC2H3O2 is combined with enough water to make a 500.0 mL solution, the concentration of HC2H3O2 is __________ M. A) 3.33 B) 1.00 C) 0.835 D) 0.00167 E) 0.150 40. What is the concentration (M) of CH3OH in a solution prepared by dissolving 16 g of CH3OH in sufficient water to give exactly 250 mL of solution? A) 11.9 B) 1.59 x 10-3 C) 0.0841 D) 2.00 E) 11.9 x 10-3 41. What is the concentration (M) of a NaCl solution prepared by dissolving 9.3 g of NaCl in sufficient water to give 350 mL of solution? A) 18 B) 0.16 C) 0.45 D) 27 E) 2.7 x 10-2 42. How many grams of CH3OH must be added to water to prepare 150 mL of a solution that is 2.0 M CH 3OH? A) 9.6 x 103 B) 4.3 x 102 C) 2.4 D) 9.6 E) 4.3 43. The molarity (M) of an aqueous solution containing 22.5 g of sucrose (C 12H22O11) in 35.5 mL of solution is __________. A) 0.0657 B) 1.85 x 10-3 C) 1.85 D) 3.52 E) 0.104 44. The molarity (M) of an aqueous solution containing 52.5 g of sucrose (C 12H22O11) in 35.5 mL of solution is __________. A) 5.46 B) 1.48 C) 0.104 D) 4.32 E) 1.85 45. How many grams of NaOH (MW = 40.0) are there in 500.0 mL of a 0.175 M NaOH solution? A) 2.19 x 10-3 B) 114 C) 14.0 D) 3.50 E) 3.50 x 103 46. How many grams of H3PO4 are in 175 mL of a 3.5 M solution of H3PO4? A) 0.61 B) 60 C) 20 D) 4.9 E) 612 2|Page 47. How many grams of sodium chloride are there in 55.0 mL of a 1.90 M aqueous solution of sodium chloride? A) 0.105 B) 6.11 C) 3.21 D) 6.11 x 103 E) 12.2 48. How many grams of sodium chloride are there in 550.0 mL of a 1.90 M aqueous solution of sodium chloride? A) 61.1 B) 1.05 C) 30.5 D) 9.6 x 104 E) 122 49. A substance is dissolved in water, forming a 0.50 molar solution. If 4.0 liters of solution contains 240 grams of the substance, what is the molecular mass of the substance? A) 60 g/mol B) 120 g/mol C) 240 g/mol D) 480 g/mol E) 640 g/mol Freezing point depression- which has the lowest? o Solutions and IMFs 68-70 68. Which of the following liquids will have the lowest freezing point? A) pure H2O B) 0.10 m aqueous KBr C) 0.10 m aqueous FeBr2 D) 0.10 m aqueous FeBr3 E) 0.10 m aqueous CaBr2 69. Of the following, a 0.1 M aqueous solution of __________ will have the lowest freezing point. A) NaCl B) Al(NO3)3 C) K2CrO4 D) Na2SO4 E) sucrose, C12H22O11 70. Of the following, a 0.2 M aqueous solution of __________ will have the highest freezing point. A) (NH4)3PO4 B) Pb(NO3)2 C) Na3PO4 D) Mg(NO3)2 E) NaCl Boiling point - what factors affect it? Comparison of substances’ boiling point based on structure. Polar vs. non polar vs. ionic. Size? o o Solutions and IMFs 70,78 IMFs 20-22 70.Which of the following aqueous solutions will have the highest boiling point? A) 0.10 m Na2SO4 B) 0.10 m glucose, C6H12O6 C) 0.10 m sucrose, C12H22O11 D) 0.10 m NaCl E) 0.10 m CuSO4 78. Which of the aqueous solutions will have the highest boiling point? A) 0.10 m Na2SO4 B) 0.20 m glucose C) 0.25 m sucrose D) 0.10 m NaCl E) 0.10 m SrSO4 20) Which one of the following should have the lowest boiling point? A) PH3 B) H2S C) HCl D) SiH4 E) H2O 3|Page 21) Of the following substances, __________ has the highest boiling point. A) H2O B) CO2 C) CH4 D) Kr E) NH3 22) Of the following, __________ has the highest boiling point. A) N2 B) Br2 C) H2 D) Cl2 E) O2 Definition of boiling as it related to vapor pressure and atmospheric pressure 39) Water could be made to boil at 105°C instead of 100°C by _____. A) increasing the air pressure on the water B) decreasing the air pressure above the water C) decreasing the pressure on the water D) applying a great deal of heat IMFs- recognizing hydrogen bonding in molecules o IMFs 12,13,14,17 12) Which of the following has hydrogen bonding as its only intermolecular force? A) HF B) H2O C) C6H13NH2 D) C5H11OH E) None, all exhibit dispersion forces. 13) Which one of the following substances will have hydrogen bonding as one of its intermolecular forces? 14) Which one of the following substances will not have hydrogen bonding as one of its intermolecular forces? 17) In which of the following molecules is hydrogen bonding likely to be the most significant component of the total intermolecular forces? A) CH4 B) C5H11OH C) C6H13NH2 D) CH3OH E) CO2 4|Page Melting point- what factors affect it? Polar vs. non polar vs. ionic. Size? o Solutions and IMFs 63,66,67,68 21) Of the following substances, __________ has the highest boiling point. A) H2O B) CO2 C) CH4 D) Kr E) NH3 22) Of the following, __________ has the highest boiling point. A) N2 B) Br2 C) H2 D) Cl2 E) O2 Solubility curves and the determination of saturated, unsaturated and supersatured solutions based on data. o Solutions and IMFs 14,15,16 14. A solution with a concentration higher than the solubility is __________. A) is not possible B) is unsaturated C) is supercritical D) is saturated E) is supersaturated 15. A supersaturated solution __________. A) is one with more than one solute B) is one that has been heated C) is one with a higher concentration than the solubility D) must be in contact with undissolved solid E) exists only in theory and cannot actually be prepared 16. A sample of potassium nitrate (49.0 g) is dissolved in 101 g of water at 100 °C, with precautions taken to avoid 5|Page evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is __________. A) hydrated B) placated C) saturated D) unsaturated E) supersaturated Heat of vaporization and its relationship to IMFs o IMFs 18,19 18) Based on the following information, which compound has the strongest intermolecular forces? A) Argon B) Benzene Substance ΔHvap (kJ/mol) C) Ethanol Argon (Ar) 6.3 D) Water Benzene (C H ) 31.0 6 6 E) Methane Ethanol (C2H5OH) 39.3 Water (H2O) 40.8 Methane (CH4) 9.2 19) Based on molecular mass and dipole moment of the compounds in this table, which has the Substance Molecular Mass Dipole Moment highest boiling point? (amu) (D) A) CH3CH2CH3 B) CH3OCH3 Propane, CH3CH2CH3 44 0.1 C) CH3Cl Dimethylether, CH3OCH3 46 1.3 D) CH3CHO E) CH3CN Methylchloride, CH3Cl 50 1.9 Acetaldehyde, CH3CHO 44 2.7 Acetonitrile, CH3CN 41 3.9 Balancing equations o Stoichiometry 1,2,3,16,23,26 1) When the following equation is balanced, the coefficients are __________. NH3 (g) + O2 (g) → NO2 (g) + H2O (g) A) 1, 1, 1, 1 B) 4, 7, 4, 6 C) 2, 3, 2, 3 D) 1, 3, 1, 2 E) 4, 3, 4, 3 2) When the following equation is balanced, the coefficients are __________. Al(NO3)3 + Na2S → Al2S3 + NaNO3 A) 2, 3, 1, 6 B) 2, 1, 3, 2 C) 1, 1, 1, 1 D) 4, 6, 3, 2 E) 2, 3, 2, 3 6|Page 3) When the following equation is balanced, the coefficient of H 2 is __________. K (s) + H2O (l) → KOH (aq) + H2 (g) A) 1 B) 2 C) 3 D) 4 E) 5 Determining the number of atoms within a compound o Stoichiometry 40,41,43 40) A 30.5 gram sample of glucose (C6H12O6) contains __________ atoms of carbon. A) 1.02 x1023 B) 6.12 x1023 C) 6.02 x1023 D) 2.04 x1023 E) 1.22 x1024 41) A sample of CH2F2with a mass of 19 g contains __________ atoms of F. A) 2.2 10 B) 38 C) 3.3 10 23 24 D) 4.4 10 E) 9.5 23 43) How many atoms of nitrogen are in 10 g of NH 4 NO3 ? A) 3.5 B) 1.5 x1023 C) 3.0 x1023 D) 1.8 E) 2 Determining the number of grams of an element within a compound o Stoichiometry 31,32 31) How many grams of hydrogen are in 46 g of CH 4O? A) 5.8 B) 1.5 C) 2.8 D) 0.36 E) 184 7|Page 32) How many grams of oxygen are in 65 g of C2H2O2? A) 18 B) 29 C) 9.0 D) 36 E) 130 53) How many moles of sodium carbonate contain 1.773 x1017 carbon atoms? A) 5.890 x10-7 B) 2.945 x10-7 C) 1.473 x10-7 D) 8.836 x10-7 E) 9.817 x10-8 Free Response Determination of an empirical formula of a hydrocarbon containing oxygen from combustion analysis data o Stoichiometry 45,46 45) A compound is composed of only C, H, and O. The combustion of a 0.519-g sample of the compound yields 1.24 g of CO2 and 0.255 g of H2O. What is the empirical formula of the compound? A) C6H6O B) C3H3O C) CH3O D) C2H6O5 E) C2H6O2 46) Combustion of a 1.031-g sample of a compound containing only carbon, hydrogen, and oxygen produced 2.265 g of CO2 and 1.236 d of H2O. What is the empirical formula of the compound? A) C3H8O B) C3H5O C) C6H16O2 D) C3H9O3 E) C3H6O3 Limiting reactant percent yield 58) Silver nitrate and aluminum chloride react with each other by exchanging anions: 3AgNO3 (aq) + AlCl3 (aq) → Al(NO3)3 (aq) + 3AgCl (s) What mass in grams of AgCl is produced when 4.22 g of AgNO3 react with 7.73 g of AlCl3? A) 17.6 B) 4.22 C) 24.9 D) 3.56 E) 11.9 8|Page 59) How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? Mg3N2 + 3H2O → 2NH3 + 3MgO A) 0.114 B) 0.0378 C) 0.429 D) 0.0756 E) 4.57 7. Molecular behavior based on structure- solubility, IMFs, vapor pressure Account for each of the following observations about pairs of substances. In your answers, use appropriate principles of chemical bonding and/or intermolecular forces. In each part, you answer must include references to both substances. (a) Even though NH3 and CH4 have similar molar masses, NH3 has a much higher boiling point (-33 ºC) than CH4 (-164 ºC). (b) At 25 ºC and 1.0 atm, ethane (C2H6) is a gas and hexane (C6H14) is a liquid. (c) Si melts at a higher temperature (1,410 ºC) than Cl2 (-101 ºC). (d) MgO melts at a much higher temperature (2,852 ºC) than NaF (993 ºC). 9|Page









