Chapter 2
advertisement

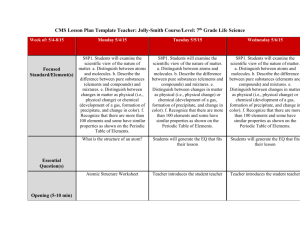
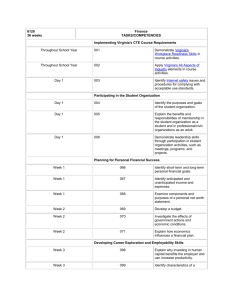
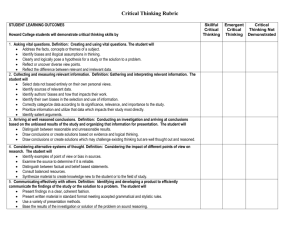
Name: _____________________________________________ Date: ______________ Period: _________ Chapter 2: Science, Matter, Energy, and Systems Vocabulary: Due Scientific hypothesis Null hypothesis Scientific theory Scientific law Peer review Inductive reasoning Deductive reasoning Paradigm shift Frontier science Matter Atomic theory Atomic number Mass number Isotope Acidity pH Organic compounds Inorganic compounds Genes Traits Matter quality Natural radioactive decay Nuclear fission Nuclear fusion Radioactive isotope Law of conservation of matter Kinetic energy Potential energy Heat Electromagnetic radiation Energy quality Law of conservation of energy Second law of thermodynamics Energy efficiency System Input Throughput Output Feedback loop Time delay Synergistic interaction Tipping point Objectives: Due 1. Describe the controlled scientific experiment carried out at the Hubbard Brook Experimental Forest. 2. Describe the steps involved in the scientific process. 3. Think of an area you have seen where some significant change has occurred to a natural system. What is a question you might ask in order to start a scientific process to evaluate the effects of this change? 4. Distinguish among a scientific hypothesis, scientific theory, and scientific law. 5. What is peer review and why is it important? 6. Explain why scientific theories are not to be taken lightly and why people often use the term “theory’ incorrectly. 7. Distinguish between inductive reasoning and deductive reasoning and give an example of each. 8. What are five limitations of science and environmental science? 9. What is the atomic theory? 10. Distinguish between the atomic number and the mass number of an element. 11. Distinguish between organic compounds and inorganic compounds and give an example of each. 12. Distinguish among complex carbohydrates, proteins, nucleic acids, and lipids. 13. Distinguish between high-quality matter and low-quality matter and give an example of each. 14. Explain the differences among natural radioactive decay, nuclear fission, and nuclear fusion. 15. What is the law of conservation of matter and why is it important? 16. A tree grows and increases its mass. Explain why this phenomenon is not a violation of the law of conservation of matter. 17. Distinguish between kinetic energy and potential energy and give an example of each. 18. Define and give two examples of electromagnetic radiation. 19. Distinguish between high-quality energy and low-quality energy and give an example of each. 20. Someone wants you to invest money in an automobile engine, claiming that it will produce more energy than the energy in the fuel used to run it. What is your response? Explain. 21. What is the second law of thermodynamics and why is it important? 22. Explain why this law means that we can never recycle or reuse high-quality energy. 23. Distinguish among the input, throughput, and output of a system. 24. Distinguish between a positive feedback loop and a negative feedback loop in a system and give an example of each. 25. Distinguish between a time delay and a synergistic interaction in a system and give an example of each.