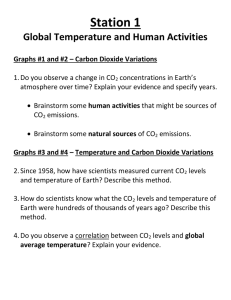
CO2 and Ocean pH
advertisement

CO2 and Ocean pH To determine the relationship between pH, atmospheric carbon dioxide and the relative concentrations of carbonate species, a detailed analysis of the equilibrium equations is required. Atmospheric carbon dioxide exists in a dynamic equilibrium with dissolved carbon dioxide, represented in equation 1: CO2(g) ⇋ CO2(aq) (1) The equilibrium expression is: K CO2 [CO2 ] pCO2 (2) where [CO2] is the concentration of dissolved carbon dioxide and pCO2 is the partial pressure of atmospheric carbon dioxide. It is important to note that equilibrium constants are dependent on the matrix in which the reaction occurs. Thus, the equilibrium constant is dependent on temperature, salinity and the presence of other ions in solution, as well as other factors. Once carbon dioxide has dissolved into the ocean, carbon dioxide molecules can react with water molecules to create carbonic acid, according to equation 3: CO2(aq) + H2O(l) ⇋ H2CO3(aq) (3) The equilibrium constant for equation 3 is very small and the majority of dissolved carbon dioxide molecules will not react. However, aqueous carbon dioxide molecules are difficult to experimentally distinguish from aqueous carbonic acid molecules,1 so we will approximate the concentration of carbonic acid (H2CO3*) to be the sum of the concentrations of both aqueous carbon dioxide and carbonic acid. With this approximation, equation 2 can be rewritten: K CO2 [ H 2CO3 *] pCO2 (4) As a diprotic acid, carbonic acid can then react twice with water to form carbonate: H2CO3(aq) + H2O(l) ⇋ HCO3-(aq) + H3O+(aq) (5) HCO3-(aq) + H2O(l) ⇋ CO32-(aq) + H3O+(aq) (6) Equations 5 and 6, respectively, have equilibrium constants: K a1 [ HCO3 ][ H 3 O ] [ H 2 CO3 *] (7) 2 Ka2 [CO3 ][ H 3O ] [ HCO3 ] (8) For the calculations in the applet, the values Ka1=10-5.831 and Ka2=10-8.915 were used.1 By applying Le Chatelier's principle to equation 1, it is clear that increasing the atmospheric concentration of carbon dioxide will affect the dissolution of carbon dioxide into the ocean. This will cause equilibria given by equations 3, 5 and 6 to shift, changing the pH of the ocean, as the hydronium ion concentration changes. However, the calculation that relates the partial pressure of carbon dioxide in the atmosphere to the pH of the ocean is quite complex and relies on a number of factors. The computer program CO2SYS was used to calculate the pH of the ocean for several partial pressures of carbon dioxide and a linear approximation was performed to link the pH to the natural logarithm of the partial pressure of carbon dioxide. This linear function was used to calculate the pH from the natural logarithm of atmospheric carbon dioxide concentration as determined by the slider bar in the applet; the function is also plotted in the graph tool, under the tab "CO2 vs pH". Carbon Speciation in the Open Ocean From the pH of the ocean, the concentrations of the various carbonate species (H2CO3*, HCO3and CO32-) can be determined. Since the concentration of hydronium ion is most easily measured as a pH value, it is more appropriate to describe equations 7 and 8 in terms of pH, rather than hydronium ion concentration. The pH of a solution is the negative logarithm of the hydronium ion concentration, so, pH log[ H 3O ] [ H 3O ] 10 pH (9) Thus, equations 7 and 8 can be rewritten: 10 pH [ HCO3 ] K a1 [ H 2CO3 *] (10) 2 Ka2 10 pH [CO3 ] [ HCO3 ] (11) In an open solution, ions are free to dissolve into or evaporate out of solution. To determine the concentrations of these ions for the “Carbonates in an Open Ocean” graph in the applet, we can inspect the equilibrium constants for their reactions. Equation 4 shows that the concentration of carbonic acid (in all of its forms) is only dependent on the partial pressure of atmospheric carbon dioxide and the equilibrium constant for the reaction. Equation 4 can be rearranged: [ H 2 CO3 *] K CO2 pCO2 (12) In the applet, equation 12 is used to calculate the concentration of H2CO3, the logarithm of which was plotted in the open system graph. The atmospheric concentration of carbon dioxide, as set by the slider, is converted to a partial pressure and substituted into this equation. In the applet, KCO2=10-1.48 is used. This value was obtained by multiplying the K value for the dissolution of carbon dioxide into pure water (Henry's Law Constant: 3.36x10-4 mol L-1 kPa-1) by the atmospheric pressure (101.325 kPa) and dividing by the density of seawater, which accounts for the difference between pure and ocean water systems.2 The density value that is used is 1.025 kg/L, which is well within measured density ranges for seawater.3 Note that the graph displays the concentration of H2CO3*, which is the sum of the concentrations of carbonic acid and dissolved carbon dioxide. However, for simplicity's sake, this plot is labelled solely as carbonic acid. It is also interesting to note that the concentration of carbonic acid is independent of the pH and changes only with the concentration of atmospheric carbon dioxide. Next, in order to determine the concentration of bicarbonate in an open system, equation 10 can be rearranged: K a1[ H 2CO3 *] 10 pH [ HCO3 ] and equation 12 can be substituted in: [ HCO3 ] K a1 K CO2 pCO2 (13) 10 pH The applet uses equation 13 to plot the logarithm of the concentration of bicarbonate ions in the open ocean. In order to determine the concentration of carbonate ions in an open system, equation 11 can be rearranged: 2 [CO3 ] K a 2 [ HCO3 ] 10 pH and equation 13 can be substituted in: 2 [CO3 ] Ka2 K a1 K CO2 pCO2 10 pH 10 pH 2 [CO3 ] K a 2 K a1 K CO2 pCO2 (14) 10 2 pH Equation 14 is used in the applet to plot the logarithm of the concentration of carbonate ions in the open ocean. Carbon Speciation in a Closed System In a closed system, we can more specifically analyze the relative concentration of each species. Since a closed system cannot lose molecules from solution, the total concentration of all species of a molecule (CT) must remain constant, even if relative amounts of species differ. 2 CT [ H 2CO3*] [ HCO3 ] [CO3 ] (15) Since equation 15 must remain a constant value in a closed system, we can consider the percent relationship between the three concentrations. For carbonic acid, this relationship is, % CT ( H 2CO3 *) [ H 2CO3 *] 100% 2 [ H 2CO3 *] [ HCO3 ] [CO3 ] (16) By substituting equations 12-14 into equation 16, we can further simplify it: % CT ( H 2CO3 *) K CO2 pCO2 % CT ( H 2CO3 *) K CO2 pCO2 100% K a1 K a 2 K a1 K CO2 pCO2 (1 pH 2 pH ) 10 10 % CT ( H 2CO3 *) % CT ( H 2CO3 *) % CT ( H 2CO3 *) K CO2 pCO2 100% K a1K CO2 pCO2 K a 2 K a1 K CO2 pCO2 10 pH 102 pH 1 K1 K K 1 apH a22 pHa1 10 10 1 1 K a1 K K a22 pHa1 pH 10 10 10 2 pH 100% 10 2 pH 100% 10 2 pH 10 2 pH 100% 10 pH K a1 K a 2 K a1 (17) By applying the same simplification method, the percentage of bicarbonate and carbonate ions in solution can be obtained: % CT ( HCO3 ) 10 2 pH 2 % CT (CO3 ) 10 2 pH 10 pH K a1 100% 10 pH K a1 K a 2 K a1 (18) K a 2 K a1 100% 10 pH K a1 K a 2 K a1 (19) Equations 17-19 show that the speciation of carbonate in a closed system is dependent only on the pH. This is a logical result, because equations 12-14 specify that the speciation in an open system is only dependent on the atmospheric concentration of carbon dioxide and the pH, but a closed system will not interact with atmospheric carbon dioxide. Equations 17-19 were used to plot the percentage of each carbonate species on the “Carbonates in a Closed System” graph, using the ocean pH as determined by the atmospheric CO2 slider. Just as in the graph for the open ocean, the plot for H2CO3* is labelled only as carbonic acid, although the plot depicts the percentage of total that is comprised of both carbonic acid and dissolved carbon dioxide. References 1 Weston, R. E. Jr. (2000). Climate Change and Its Effect on Coral Reefs. J. Chem. Educ., 77(12), 1574-1577. 2 Mahaffy, P. G.; Bucat, B.; Tasker, R.; Kotz, J. C.; Treichel, P. M.; Weaver, G. C.; McMurry, J. Chemistry: Human Activity, Chemical ReactivityFirst Canadian Edition; Nelson: Toronto, 2011; p. 465. 3 CRC Handbook of Chemistry and Physics, 91st ed.; Haynes, W. M., Ed.; CRC Press: Boca Raton, FL, 2010; Section 14, No. 16. Acknowledgements Dr. Kris Ooms (The King's University College) for offering input and providing resources concerning carbon speciation.