Notes: Water
advertisement
Water
Biol 135 Lecture:
VI. Structure, Function and Source of Micronutrients.
A. Water
Water is Essential and the Basis to Life
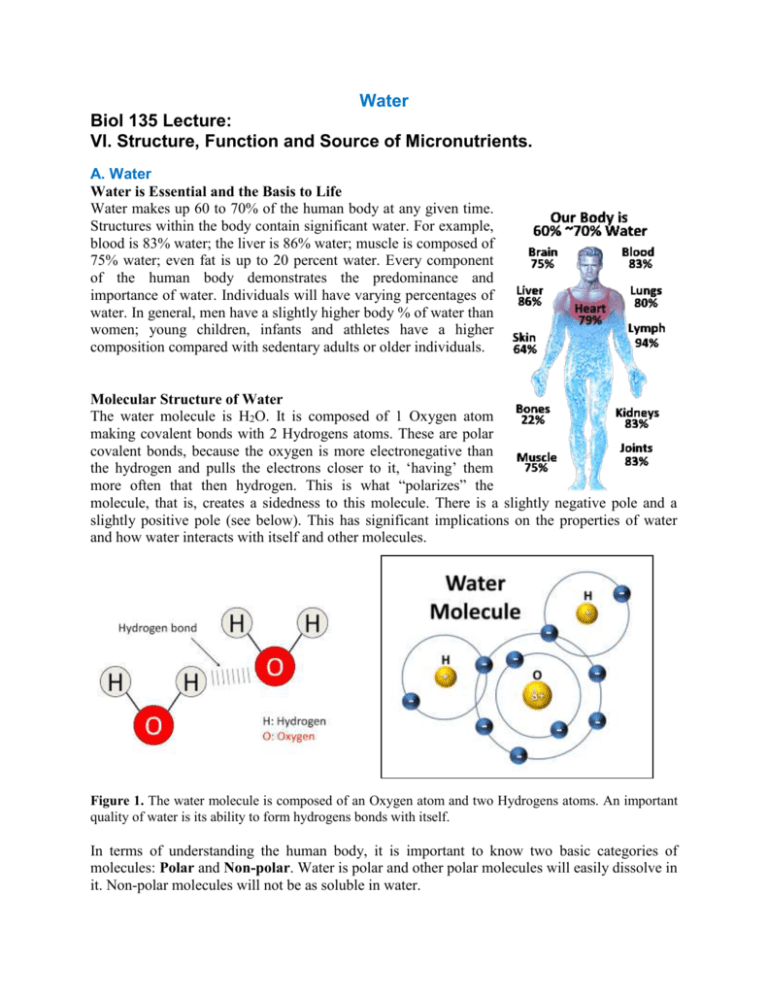
Water makes up 60 to 70% of the human body at any given time.
Structures within the body contain significant water. For example,
blood is 83% water; the liver is 86% water; muscle is composed of
75% water; even fat is up to 20 percent water. Every component
of the human body demonstrates the predominance and
importance of water. Individuals will have varying percentages of
water. In general, men have a slightly higher body % of water than
women; young children, infants and athletes have a higher
composition compared with sedentary adults or older individuals.
Molecular Structure of Water
The water molecule is H2O. It is composed of 1 Oxygen atom
making covalent bonds with 2 Hydrogens atoms. These are polar
covalent bonds, because the oxygen is more electronegative than
the hydrogen and pulls the electrons closer to it, ‘having’ them
more often that then hydrogen. This is what “polarizes” the
molecule, that is, creates a sidedness to this molecule. There is a slightly negative pole and a
slightly positive pole (see below). This has significant implications on the properties of water
and how water interacts with itself and other molecules.
Figure 1. The water molecule is composed of an Oxygen atom and two Hydrogens atoms. An important
quality of water is its ability to form hydrogens bonds with itself.
In terms of understanding the human body, it is important to know two basic categories of
molecules: Polar and Non-polar. Water is polar and other polar molecules will easily dissolve in
it. Non-polar molecules will not be as soluble in water.
2
Figure 2. The polarity of the water molecule gives it a sidedness in terms of electrical charge.
The Many Functions of Water in the Body
Since our body is about 70%water, it is the most abundant single component of the body! As
such, it is involved in every aspect of how our body functions and the most significant way we
get our water is through our diet. Outlined briefly below are some general functions of water in
the human body.
Transportation
The blood that travels through our blood vessels and the lymph in lymphatic vessels is the
primary way that substances are moved throughout our body. The transportation of nutrients
throughout the body occurs based predominantly on what is dissolved in the plasma - the watery
portion of blood. The plasma also transports the gases O2, and CO2around the body and
hormones to and from cells.
List of Substances Dissolved in Plasma
Plasma Proteins (mostly albumins), ions
(electrolytes), minerals, hormones, vitamins,
fatty acids, triglycerides, lipoproteins (VLDL’s,
LDL’s, HDL’s), glucose, amino acids, dissolved
gases O2, and CO2, antibodies, enzymes,
eicosanoids and various metabolic waste
products such as urea and uric acid.
Water is necessary in order to remove the waste products normally generated by your body.
Referred to as ‘metabolic wastes’. We always need to lose some water in the processes of
excretion of fluid waste that occurs in the kidneys called Urine and solid waste generated by the
gastrointestinal tract called Feces.
Water plays an important role in the Digestion of nutrients in the body. Water is required for the
breakdown (Hydrolysis) of the 3 organic macronutrients that are energy yielding.
3
Water helps to maintain stable body temperature (Tb)–It is especially crucial for the regulation
of Cooling the body down!
Water is an important Lubricant for many areas of the body that are either heavily used (like
joints, internal body cavities and muscles) or exposed to the outside environment:
•
•
•
•
•
•
Joints
Internal Body Cavities
Eyes
Mouth and Gastrointestinal Tract
Lungs
Urogenital Tract
Protective Cushion
Water anywhere in the body provides an insulative cushion that protects us against physical
trauma. Also, in Pregnancy, the fetus is surrounded by watery amniotic fluid and this offers
protection and insulation.
Water provides the Structure to Cells – the intracellular fluid (inside the cell) and the
extracellular fluid (outside the cell) are mostly water and contribute to the shape and structure of
all cells in the body. Water also provides structure and bulk to muscle cells, for example. This is
especially true under the influence of a carbohydrate-loading diet and the generous storage of
glycogen in muscle cells.
The 4 Properties of Water are:
1) Solvency – the Universal Solvent.
2) Cohesion – has a high affinity for itself.
3) Thermostability – has a very stable temperature.
4) Reactivity – participates in chemical reactions in the body.
Water is a Universal Solvent and Transport medium. It is a polar molecule and many things can
make H-bonds with it and dissolve in solution. It contributes to the dissolving of particles,
digestion, and nutrient and waste transportation. The polarity of water attracts charged particles
4
and dissolves other polar substances, including proteins, glucose, and many minerals. The water
in blood allows for the transportation of oxygen (O2), nutrients, and hormones to all cells in the
body. Since everything we eat and absorb goes into our blood stream, the types of food we eat
will have a significant impact on the water in our body. Water is essential to maintaining acidbase balance in the body, and the various foods that we eat will also have an impact on the pH of
our body fluids (which of course are mostly water).
Water Helps Maintain Body Temperature
Heat is absorbed in the body’s internal core and carried to the skin for release. Water has a high
heat capacity (specific heat), allowing it to absorb and hold heat longer with little change in
temperature.
Water has a High Heat Capacity: This means it takes a lot of heat energy to change the
temperature of water.
Water has a High Heat of Vaporization: This means when water goes from a liquid to a gas, it
takes a lot of heat energy with it.
When our internal body temperature rises higher due to physical activity or other factors, the
increased heat breaks down hydrogen bonds, transforming the water from sweat to vapor,
releasing the heat and further cooling the body. As we know, when water goes from a liquid to a
vapor form it takes a lot of heat energy with it and leave you cooler.
The watery fluids, like the tears in your eyes and the saliva in your mouth are ~ 95% water, but
they also contain oils, proteins and antimicrobial agents (like lysozymes). Interestingly, human
milk and mucus have significant water and also contain antimicrobial agents and
immunoglobbins to protect us.
Water Participates in Chemical Reactions: Dehydration Synthesis (Condensation) and
Hydrolysis Reactions.
The three energy yielding organic macronutrients that we eat - Carbohydrates, Lipids and
Proteins – all require water to hydrolyze their covalent bonds that hold these molecules together.
5
This occurs during digestion. As the body uses ingested nutrients to make other molecules, water
is pulled out of these molecules by dehydration synthesis.
When we consume polymers of any of these organic nutrients, they undergo Hydrolysis, this
involves an addition of a hydrogen ion to one molecule and a hydroxyl group to the other end of
the newly formed molecule. This releases the energy stored in the chemical bonds as the nutrient
is metabolized. When our bodies are combining smaller molecules to make a larger, more
complex molecule, it does so removing a water molecule is from every bond that is formed when
adding more ‘pieces’ to the larger molecule. Energy is being stored in the new covalent bonds
that are made through this process called Dehydration Synthesis.
Water plays a role in Acid-Base Balance
Because water is polar, it can be used to decrease or increase pH levels by either breaking down
or forming carbonic acid. That is, it can act as either an acid or a base.
What is the body's pH?
As we know, water is the most abundant compound in the human body. The various
compartments of the body have different ranges of pH’s, depending on their primary function,
some may be more or less acid.
The term pH means “potential of Hydrogen” it is actually a measure of the [H+] (reads: hydrogen
ion concentration) in a fluid by taking the negative of the logarithmic scale! This is done so that
the numbers are not 0.000001 but are between 0 and 14 and are easy to compare.
The higher the pH reading, the more alkaline (or basic) and oxygen rich the fluid is.
The lower the pH reading, the more acidic and oxygen deprived the fluid is.
The pH range is from 0 to 14, with 7.0 being neutral. Anything above 7.0 is alkaline, anything
below 7.0 is considered acidic. Going up or down one number, say from 7 to 6, represents an
order of magnitude difference, in other words the solution going from 7 to 6 just got 10 times
more acidic! If it does to 5 it is now 100 times as acidic as the solution at 7.
The pH of human blood is slightly alkaline and should be within the range of 7.35 - 7.45. Below
or above this range means unhealthy symptoms and disease. If blood pH moves below 6.8 or
above 7.8, cells stop functioning completely and the body dies! The body therefore continually
strives to balance pH. When this balance is compromised many problems can occur.
6
The most common imbalance in the American diet is a condition called acidosis, when the pH of
our body fluids fall below the low end of that safe range. For example a blood pH of 7.30 creates
a condition of metabolic acidosis, even though technically that value is still an alkaline solution
(above 7.0), it is acidic as far as the homeostatic mechanisms of the human body are concerned
and your systems will strive to get your pH back into the normal range (7.35 to 7.45).
A diet high in acidic-producing foods such as sugar, alcohol, highly processed foods and high
levels of caffeine (see Table 1) puts pressure on the body's regulating systems to maintain pH
neutrality. The extra buffering required can deplete the body of alkaline minerals such as sodium,
potassium, magnesium, and calcium, making the person prone to chronic and degenerative
disease. Minerals are borrowed from vital organs and bones to buffer (neutralize) the acidic
conditions and safely eliminate the excess acids from the body. Because of this strain, the body
can suffer severe and prolonged damage.
Health Problems Caused by Acidosis
Research shows that unless the body's pH level is slightly alkaline, the body cannot heal itself.
So no matter what means you choose to take care of your health, it won't be effective until the
pH level is balanced. If your body's pH is not balanced, for example, you cannot effectively
assimilate vitamins, minerals and food supplements. Your body pH affects everything.
Acidosis will decrease the body's ability to absorb minerals and other nutrients, decrease the
energy production in the cells, decrease its ability to repair damaged cells, decrease its ability to
detoxify heavy metals, and make your body more susceptible to fatigue and illness. In addition,
these kinds of conditions are ripe for caners and make tumor cells thrive.
Table 1. A general list of foods and their estimated Acid or Alkaline forming tendencies.
Strongly
Acidic
pH 4.0
White Bread,
Processed
Cheese
Alcohol, Beers
Mildly Acidic
pH 4.5 to 6.5
Neutral
pH 7.0
Mildly Alkaline
pH 7.5 to 9.5
Strongly Alkaline
pH 10.0
Meat and Fish,
Canned Fruits
De-ionized
Water
Many Fruits,
Coconut
Melon, Raisins,
Vegetable Juices
Legumes
Egg Yolks
Most Vegetables
Asparagus, Garlic
Sodas,
Most Nuts
Raw Milk
Almonds
Cayenne Pepper
Tea, Coffee
Cucumber
Sugar,
Cooked Spinach Raw Honey
Avocados
Raw Spinach
French Fries
Kale
Peanuts,
Brown Rice
Basmati Rice
Quinoa, Buckwheat Kelp, Celery,
Walnuts
Broccoli
Dairy, Cocoa
Raw Cream,
Falx Seed
Raw Swiss Chard
Artificial
Whey
Olive oil
Sweeteners
Note: There are various discrepancies between the acid- or alkaline-forming values given in the
numerous lists provided by many websites and references. The table above is not to be taken as
anything more than an approximate guide. We have many sources of informationat our fingertips
7
and there are always likely to be errors or misconceptions in any format. From the many
websites I’ve examined, the information compiled is like an amalgamation of the more common
themes I have encountered. Many aspects are from sources that have cited personal experience
and experience with clients, literally measuring body fluids with litmus paper before and after
cleansing treatments. There has been a wealth of information gathered over time, much of which
has not involved the scientific laboratory testing of isolated substances. This does not invalidate
the positive results that can be seen in other types of studies. Often the physical, mental and
spiritual transformation of an individual is quite a powerful piece of evidence.
The basic principles of good health seem remarkably clear: Eat Good Food.
Perhaps the issue for many people is that they may be uncertain as to what good food is. Could
that be true? OR, is it that most people know what qualifies as good food but are unwilling to
change the habits they have become accustomed to for most of their lives, regardless of their
state of health?
The point of a class like this one is to present the best type of information so that you can make
informed decisions about what you eat. The best information really means the most accurate –
that is, the closest to the actual truth. The truth is often hiding in plain sight for all to see, if you
are looking for it! Accurate information and thoughtful advice does not necessarily come from
the big “trusted” icons of health, those amazing experts who would like you to believe that they
know more than you do and only they know what is best for you. Strangely enough, finding the
truth can sometimes come from your own experience. The best piece of advice I can give anyone
is to dig deep and examine as much material as you can about a topic in nutrition that interests
you. Be open-minded about what you might find, as it may be contrary to everything you have
ever heard! Read carefully the many scientific and highly regarded studies that are out there to be
found when it comes to any issue that is not in agreement with ‘conventional wisdom’. Often it
takes quite a while for the real, truthful information to gain acceptance. This is partly because
any ‘myth’ that is repeated over and over and over has become a belief for many (even
regardless if it is a lie), and some people do not want to change their minds about anything! The
other reason may be that many entities have a vested interest in the lie being perpetuated. That is,
many actually benefit by others being poorly informed and making poor decisions about their
health. They gain from your sickness – pretty terrible huh!? If you know the truth and still want
to eat “Froot Loops” for breakfast, knock yourself out! The number one ingredient is sugar plus a
host of artificial flavors and colors; notice the word Fruit is not used for a reason, no fruit item
has come within a mile of that box – and they don’t want to lie to you after all! However, if you
don’t want to be a victim, then educate yourself, it’s really not that hard to do. Examine all the
information with an open mind and positive attitude and do not be afraid to go with your
intuition – especially if you have practiced the art of trusting it!
So here is some truth about the good food to eat.
Eat whole foods. Basically anything that comes right out of the ground and you can eat directly!
What this means is that a good diet that will keep you healthy should contain a significant
portion of fresh organic vegetables and some fruit daily. Try not to eat too many grain products,
keep to whole grain and especially avoid the refined grains – that is, like the ones found in most
8
breakfast cereals. They can be almost as bad as that highly addictive toxic substance called
Sugar! If you know that your body does well with dairy products, then see if you can incorporate
some raw dairy, like raw milk and raw cheese. It is highly nutritious because without the high
temperature of pasteurization many more of the vital nutrients are retained to benefit you.
Getting high quality protein directly from eggs, meat and fish is also recommended. If you are
vegetarian, there are many nutrient dense whole grains (like quinoa, brown rice and
buckwheat) legumes and nuts and many other viable options. Vegans (who do not eat any
animal products) will need to take supplements, as there are several essential nutrients that your
body must have to function optimally that can only be obtained from animals.
With regard to the acid- and alkaline-forming foods please remember, you don't have to cut out
all acid-forming foods - some are necessary, typically 40% - otherwise you probably wouldn't
get enough protein and variety of nutrients, yet alone make interesting meals that you enjoy. It is
advisable to shift the overall balance of your diet over toward the alkaline, and away from the
excessively acid-forming diet of our ‘quick-food culture’.
Please note the obvious major difference between strongly acidic and strongly alkaline foods in
Table 1. The strongly acidic foods are highly processed and the strongly basic foods are nutrient
rich whole food vegetables (not fruits!).
Foods: Are they Acid or Alkaline-forming? Note that a food's acid or alkaline-forming
tendency in the body has nothing to do with the actual pH of the food itself. For example, lemons
are very acidic, however the end-products they produce after digestion and assimilation are
alkaline so lemons are alkaline-forming in the body. Likewise, meat and dairy products will test
9
alkaline before digestion but it leaves acidic residue in the body so, like nearly all animal
products, meat and dairy are classified as acid-forming.
It is important that your daily dietary intake of food naturally acts to balance your body pH. To
maintain health, the diet should consist of at least 60% alkaline forming foods and at most 40%
acid forming foods. To restore health, the diet should consist of 80% alkaline forming foods and
20% acid forming foods.
Free range eggs, fish, beans, healthy fats - these are healthy foods, low glycemic and nutritious,
and even if marginally acid-forming, or alkaline or in between the two depending on how you
measure or what chart you read! These food substances are not the culprits in an acid-forming
diet. The real culprits are refined sugar, highly processed foods (pastries, breads), sodas,
processed meats and alcohol. These are the items to reduce in your diet or to simply cut out of
your diet entirely. Away! Not only is consuming refined food in and of itself setting your body
into a poor condition, but this food is replete with artificial things. These include synthetic
stimulants, artificial colors, artificial flavors, artificial sweeteners, preservatives, pesticides,
genetically modified sources and more - all of which are undesirable for your good health. These
are important issue we will continue to explore in this course.
Beginning to make more alkaline food choices is not too hard. For example it is better for you to
have brown rice rather than white rice, even though eating that white rice may make you feel
very happy, as it is something you have always done. Give brown rice a try. Both types of rice
are on the acid-forming side, but the brown rice less so, so that will move you in the right pH
direction (less acidic). For many other reasons aside for the pH forming issue, whole brown rice
is more healthy and nutritious choice.
Detoxify with Vegetable & Fruit Juices
All natural, raw, vegetable and fruit juices are alkaline-producing. Fruit juices become more
acid-producing when processed and especially when sweetened – so avoid that! Fruit juices
when mixed with vegetable juices make for a perfect taste and health benefit combination.
The Science: Why are acidic lemons alkaline-producing?
The answer is simply that when we digest the food, it produces alkaline residue. That's why we
classify it as an alkaline food. When we digest a food it is chemically oxidized ('burned') to form
water, carbon dioxide and an inorganic compound. The alkaline or acidic nature of the inorganic
compound formed determines whether the food is alkaline or acid-producing. If it contains more
sodium, potassium or calcium, it's classed as an alkaline food. If it contains more sulfur,
phosphate or chloride, it's classed as an acid food.
What difference does it make to have toxic blood?
In summary and at the risk of sounding repetitive, an acidic body pH is not good for your health.
This can occur from an acid-forming diet, emotional stress, toxic overload, and/or immune
reactions or any process that deprives the cells of oxygen and other nutrients. The body will try
to compensate for acidic pH by using alkaline minerals. If the diet does not contain enough
minerals to compensate, a build-up of acids in the cells will occur. When you're over-acid your
body will store excess acid in your fat cells (which is why many people have trouble losing
10
weight). Over time, your body will leach calcium and alkaline stores from your bones in a
desperate attempt to retain the pH balance in your body. This can lead to osteoporosis and other
maladies. Your blood plays a very important role in your healthy and energy: it carries oxygen to
all your cells! This gives you energy, and it's what keeps you alive. It also plays a key role in
how your sleep patterns can help energize you, instead of waking up tired!
Body Water and Osmolarity
There are several ways to measure the concentration of solutes in a solution, and Osmolarity is
common way of measuring the concentration of solutions in the human body. Osmolarity is
defined as the number of “particles” (solutes) per litre (L) of solution and is called “osmoles”
(Osm) and expressed asosmol/L or Osm/L. In Physiology the very common to express
Osmolarity in milliosmoles, with values like plasma = 308 mOsM.
The molarity of a solution is expressed as “M” (pronounced “molar”) is a measure of the number
of moles of solute per unit volume of solution. The benefit of expressing body concentration in
osmoles is that you can add the values of various solutions together without having to do
separate calculations regarding the specific molecular weight of a molecule.
Example:
Osmolarity: 30 mOsM of NaCl + 20 mOsMopfGlucose = 50 mOsMNaCl Glucose solution
Molarity:
30 M of NaCl + 20 M of Glucose
≠50 M NaCl Glucose solution
You cannot add the Molarities of different solutions!
Osmolality is very similar to but different from Osmolarity
There is another term called Osmolality, with an ‘l’ rather than an ‘r’ in there! Osmolality is
osmolar concentration proportional to the number of particles per kilogram of solvent (not per
liter like Osmolarity). The distinction is made because water changes its volume with
temperature; osmolarity uses l.0 Liters (l) of water whereas osmolality uses 1.0 kg of solvent.
However, if the concentration of solutes is very low, osmolarity and osmolality are considered
equivalent, as one liter of a dilute aqueous solution at normal body temperature has a mass of
very nearly one kilogram.
Osmosis and the Tonicity of a Fluid
The term ‘tonic’ means ‘strength’, so the tonicity of a solution refers to how strong tat solution is
in comparison to another.
Osmolarity and Tonicity are related but distinct concepts. Both compare the solute
concentrations of two solutions separated by a membrane. However, Tonicity takes into account
the total concentration of non-penetrating solutes only. Non-penetrating solutes cannot cross the
cell membrane, and therefore osmosis of water must occur for the solutions to reach
equilibrium.
11
Penetrating solutes exist and are important because they can diffuse through the cell membrane,
causing momentary changes in cell volume as the solutes "pull" water molecules with them. For
our basic discussion here, we will focus on tonicity and simply ask ourselves: which direction
would water go?
Osmosis
This is a fundamental concept in living organisms! It describes the passive (no energy required)
movement of water across a semipermeable membrane - like a cell membrane.
Definition: Osmosis is the net movement of water across a semipermeable membrane from high
water concentration to low water concentration.
Any substance going from high to low concentration is doing so passively, that is, it does not
require the investment of energy. Like a ball at the top of a hill, once you nudge it, it will roll
down the hill without any effort on your part. If you want the ball to go uphill, that required
energy input, In the body, if a substance is travelling up or against its concentration gradient, it is
called an “active” process and requires energy input in the form of ATP,
If water can cross a cell membrane and will always go to where it is less, then it becomes
important to maintain the optimal tonicity of body your fluids, such that the fluid bathing your
cells has the same tonicity as the intracellular fluid inside of the cells.
Figure 3. Shows examples of three solutions with various tonicities. The solution in the beaker represents
the fluids in the body. The cell that is bathing in these solutions is affected by the tonicity of the solution,
because water (whether inside or outside of a cell) will always go to where it is less.
An Isotonic solution is one that has the same ‘concentration’ of solutes in the solution as the
fluid that is inside the cell.
This creates the perfect balance, because although water is constantly moving into and out of the
cell across the plasma membrane, it is doing so at equal rates. There is no net movement of water
in either direction. This is the picture of equilibrium and homeostasis.
A Hypertonic solution is one that has a higher ‘concentration’ of solutes in the solution
compared to the fluid that is inside the cell. This causes the water in the cell to move out of the
cell and into the solution, because the solution has less water in it (is more salty) compared to the
12
solution inside the cell. This is not good for any cell, as they begin to shrink (crenate) and can no
longer function properly.
A Hypotonic solution is one that has a lower ‘concentration’ of solutes in the solution compared
to the fluid that is inside the cell. This causes the water in the solution to move into the cell from
the solution, because the solution has more water in it (is less salty) compared to the solution
inside the cell. This is also not good for any cell, as they begin to swell and could burst (lyse) as
a consequence of being in a solution that is not strong enough
A solution can be both hyperosmotic and isotonic. For example, the intracellular fluid and
extracellular can be hyperosmotic, but isotonic – if the total concentration of solutes in one
compartment is different from that of the other, but ions cannot cross the membrane, it cannot
draw water with it, thus causing no net change in solution volume.
How Is Water Balance Maintained?
Water intake and water output are maintained in a delicate balance by the body. The body is in
water balance when the amount of water consumed is equal to the amount of water excreted.
Most Water Intake is From the Diet
Beverages are the main source, and some water is also consumed from food sources. Except for
fat, all food contains some water. A small amount of water is produced via metabolism
(metabolic water). As it turns out, 100 grams of carbohydrate can generate almost 55 grams of
metabolic water. Water joined with glucose during glycogenesis (the making of glycogen from
glucose) is released when glycogen is hydrolyzed to produce glucose. An average of 2 quarts of
water are consumed or produced by the body each day.
Water input sources (food, beverages, and the water of metabolism) and water output routes
(urine, sweat, stools, and breathing losses).
Water Excretion
Water is excreted through the kidneys, large intestine, lungs, and skin.
The majority of fluid lost is through the kidneys (via urine) at about 1,500 milliliters per day.
These losses are subject to variation based on fluid intake—the more water consumed, the more
water lost, and vice versa. Similarly, a small amount of water (about 100 milliliters) is lost
through intestinal fluid in the stool. This amount can vary if a large amount of fiber is
consumed or if a person experiences diarrhea on a regular basis. Excess water loss through
diarrhea or vomiting can lead to dehydration.
Insensible Water Loss
This is water loss due to the water that evaporates during exhalation and is lost through the skin
as the body releases heat—takes place throughout the day.
Exhaled air releases about 200 to 400 mls of water per day, but this can vary based on climate
and physical activity.
13
Water lost in sweat is not considered part of insensible water loss, as this varies greatly
depending on environmental factors and physical activity level.
Body Water is found in the Three Tissue Fluid Compartments.
There are 3 tissue compartment volumes in the body – they are all interconnected and water
moves from one compartment to the others constantly. The 3 compartment are very different to
each other in the other ions and proteins they contain. The differences in these compartments
must be constantly maintained, because this is how the body does work! In physiology, the
definition of work is simply moving things! And Energy represents the potential to do work!
The sodium potassium pump (Na+/K+ Pump) described briefly below is a key element in
maintaining the important differences that exists between the 3 compartments.
The 3 Fluid Compartments are:
1. Intracellular fluid (ICF) - inside cells contains K+, Pro-‘s and various organic acids.
2. Interstitial Fluid - bathes cells but does not circulate throughout the body, mostly Na+, Cland HCO3- solutions. Interstitial fluid makes up about 75% of ECF and acts as an area of
exchange between blood fluids and cells. Reminder - this is an Extracellular Fluid (ECF).
3. Intravascular Fluid - found in the blood and lymph vessels, the most notable being plasma,
the fluid component of blood. Reminder - this is an Extracellular Fluid (ECF).
Please note that 2 and 3 are both Extracellular Fluids (ECF). So there are two types of
extracellular fluid; one is the fluid that is in between cells in tissues (= interstitial fluid) and one
that is inside vessels (= vascular fluid).
What are Electrolytes?
Electrolytes participate in fluid balance. Some minerals act as electrolytes (electro = electricity,
lytes = soluble), or charged ions that conduct electrical current. If they have a positive charge
they are classified as cations, while negatively charged ions are called anions.
The concentration of solutes on either side of a cell membrane affects the movement of water
from a dilute concentration to a more concentrated area. Osmosis (this diffusion of water)
functions to help the concentration of solutes reach equilibrium.
Water moves through a selectively permeable membrane from an area of low concentration to an
area of higher concentration for the purpose of restoring balance to the solution. The osmolality
means the concentration of solutes in a solution; the osmotic gradient describes the ratio of
solutes on either side of the membrane. The membrane swells under the pressure of the increase
in water, or osmotic pressure.
The Sodium-Potassium Pump (Na+/K+ Pump)
In the body, the distribution of solutes and therefore water is constantly regulated. The SodiumPotassium pump maintains the volume of fluid within a cell. This system works through cell
membrane proteins to achieve stability and a homeostatic cell environment, preventing buildup
14
of solutes and water to keep cells from swelling and bursting under pressure. Sodium ions are
transported out of a cell while moving potassium ions into the cell; this drives water out of the
cell because wherever sodium goes, water follows. This pump is especially active on muscle and
nerve cells. The overall transport of ions changes the cell’s electrical charge and produces a
change of concentration; it is the driving force behind the absorption of several liters of fluid
each day plus the water secreted into the GI tract in gastric juices.
Figure 4. Shows the sodium-potassium pump that is embedded within the plasma membrane. It is a
proteins transporter that every living cell in the body requires to stay alive! It constantly experts 3 Na +
ions into the ECF and imports 2 K+ ions into the ICF using 1 ATP per cycle. It helps to maintain the
resting membrane potential (RMP) of living cells.
Proteins Regulate Fluid Balance in Body
Proteins, especially albumin, play a major role in keeping water dispersed evenly between the
ECF and the ICF. Adequate protein intake is essential to fluid balance. A diet deficient in protein
could result in accumulation of water in the interstitial spaces and swelling of body tissues,
called edema. Remember how the children suffering from protein deficiencies had distended
bellies? That was asceties, created by an accumulation of fluid in the peritoneum (abdomen) due
to lack of protein in the plasma.
How is Water Regulated?
Too Much Water in the Body
Any water you consume in your diet will go directly into your blood stream. The addition of
volume to your closed circulatory system will have the effect of increasing your blood pressure.
Like a water balloon that you fill up with more water, the pressure will increase. If you have too
much water it is relatively simple to handle. The kidneys will excrete the additional water in the
form of urine. Problem solved. There are complications if your system is out of balance and
cannot detect increases in pressure.
Too Little Water in the Body
This is a much more significant problem in the body, because you cannot make a lot of water,
and so when you are dehydrated the most important thing you body can do is to conserve water.
15
The kidneys are the key organ when it comes to conserving you body water and preventing
unnecessary water loss. One of the significant consequences of decrease water in the body is
low blood pressure. Hypotension (abnormally low blood pressure) can be very dangerous and it
is easy to lose consciousness if blood pressure suddenly drops too low.
Hormones play a very important part in water regulation of the body. The kidneys start the
cascade of water saving strategies by releasing a hormone/enzyme call renin when they detect
that your blood has too little water (it becomes a hypertonic solution!)
Renin: Released from the kidneys it catalyzes an important reaction in the blood, converting the
inactive protein angiotensinogen into angiotensin I. This is shown below by the first arrow. This
sets the stage for Angiotensin Converting Enzyme (ACE) to convert angiotensin I into
angiotensin II, which will now become the primary stimulator for water conservation
mechanisms in the body.
Angiotensinogen
(inactive)
Angiotensin I
(inactive)
Angiotensin II
(active)
The Actions of Angiotensin II
The activated hormone does the following:
1) Triggers the release of Antidiuretic Hormone (ADH) also called vasopressin, from the
posterior pituitary gland in the brain. This causes water pores to be inserted into the
collecting ducts of renal tubules and produces a more concentrated urine (containing
much less water). All you have to really know is that ADH makes your kidneys keep
more water.
2) Trigger the adrenal glands to release Aldosterone, another hormone which signals
sodium in the kidneys. Once you start retaining sodium (rather than eliminating it, you
will also retain more water, hence water retention is also a consequence of aldosterone.
3) Angiotensin II is also a potent vasoconstrictor that narrows blood vessels and raises
blood pressure.
4) Angiotensin II triggers the thirst mechanism in the hypothalamus of the brain.
In all these way, the release of renin by the kidneys helps the body reabsorb (keep) water and
salts and helps regulate blood volume and therefore blood pressure.
How Much Water Do You Need and What Are the Best Sources?
The water requirements of each individual are somewhat unique and vary from day to day
depending on physical activity and environmental factors (including diet, air temperature, and
other factors). Daily water intake recommendations are based on an average of reported water
16
intake of healthy Americans. Fruits and vegetables contain the most water on a percentage basis
when comparing solid foods.
The debate about tap water versus bottled water continues. Both tap water and bottled water can
be toxic or safe to drink, depending on where you live and the company that bottles the water!
The choice comes down being informed about the state of your water supply and knowing what
information can be withheld from you on a Nutritional Label.
The Nutrient Content of Enhanced Waters. We can discuss this in our sports drink analysis
section.
Diuretics
Diuretics are any substance that causes an increase in urine output that is not due to metabolic
activity. Consuming diuretic can contribute to water loss from the human body. Alcohol and
caffeine are considered diuretics; overconsumption can lead to fluid imbalance. Depending on
the dose or amount consumed, caffeine typically does not cause significant loss of body water.
There is a significant differences between beverages that have caffeine in them naturally as a part
of the whole food – such as coffees and various teas – to those beverages that have synthetic
caffeine added to them as a stimulant – such as in sodas and ‘energy’ drinks.
Alcohol is Dehydrating
Alcohol inhibits ADH, potentially inducing urination in as little as 20 minutes after alcohol is
consumed. Alcohol can affect the concentration of electrolytes in the body. The loss of
potassium, other mineral and water contributes to the ill effects of a hangover. Perhaps due to
tolerance, older drinkers tend to overcome the suppression of ADH somewhat faster than
younger drinkers. Reducing the amount of alcohol consumed and drinking water can help
prevent dehydration.
With regard to diuretic medications , it is my opinion that they are not the best idea in the first
place. Maybe a better approach would be to treat the hypertension in some other way that will
not create an entire host of additional ‘side effects”. Like the “Hole in the Bucket” analogy, the
best approach is to fix the problem, not suppress the symptoms! As it turns out, if someone you
know is on diuretic medications (which decrease your blood volume and in this way lowers your
blood pressure), a common side effect of prescription diuretics is an increased loss of potassium
(potentially leading to hypokalemia, or a dangerously low level of blood potassium). In many
cases, individuals are told to consume potassium-rich foods when they are taking a diuretic drug.
What would be good examples, I know you are going to say bananas! According to most
calculations, bananas are #10 on a list of foods that provide potassium (K+) in your diet. Beans,
spinach dried apricots and squash have more K+ in them than bananas.
What Are the Effects of Too Much or Too Little Water?
Water Intoxication
Consuming too much water can cause Water Intoxication. The most common and dangerous
consequence of drinking far too much water is hyponatremia. This word translates to “lower
than normal sodium levels in the blood”. This can occur by consuming too many liquids too
17
quickly without adequate sodium replacement. The effect is that your plasmasodium levels
become depleted and this also causes an increase in the rate of urine production. Having
adequate sodium levels is absolutely critical to health, the sodium ion (Na+) is fundamental to
nervous communications called action potentials, and this will adversely impact all your body
systems.
If you overload on water, there will be a decrease in the tonicity of body fluids, this will cause
tissues to swell with excess fluid, and the concentration of sodium in the extracellular fluid
drops, resulting in hyponatremia.
This state used to be fairly common among marathon runners who would rehydrate with water
only, instead of replenishing with fluids containing electrolytes. If during any strenuous physical
activity you lose many electrolytes (salts) in your sweat, if you only replace that lost volume
with water, you will be lacking in electrolytes, particularly sodium. This is the reason why for a
long time now they give marathon runners cups of fluids with plenty of electrolyte, not just
water.
As a result of hyponatremia, there is swelling of the brain due to this water intoxication and it
presents itself as fatigue, confusion, and disorientation and can lead to death. If you know or
suspect that someone is hyponatemic, a fairly quick remedy would be to give them a very ‘salty’
solution to drink! Consuming too little water is a common problem and it can become serious if
it continues unidentified.
Dehydration can result from a mere 2% loss of body water, leading to a loss of short-term and
long-term memory, lower attention span and cognition, reduced ability to maintain core
temperature, increased risk of urinary tract infection, and fatigue. For a 70kg man (about 150 lbs)
the total volume of blood is about 5.0 liters (about 1.25 gallons), so 2% of that is about 100 ml of
water. To put things into perspective, a can of soda is 355ml of fluid, so the loss of less than one
third of a soda of water from your body can result in dehydration.
Some population groups, such as children, the elderly, and athletes, are especially vulnerable to
the consequences of dehydration. Athletic performance can be hindered when very small
amounts of water are lost from the cells.
The thirst mechanism signals dehydration. The reactions that occur as part of the thirst
mechanism. The body fights dehydration as much as possible through shifts in body water
content, but the system is imperfect and limited. Fluid levels cannot return to normal without the
intake of fluids. Monitor water intake to avoid over-hydration and dehydration. Body weight is
one way to monitor water loss. A pint of water equals one pound of body weight.
By monitoring weight before and after physical activity, the appropriate amount of water can be
consumed to replace the lost fluids. For every pound of weight lost immediately following
activity, 16 fluid ounces of water should be consumed.
18
Urine Color is a Guide for Water Loss and Hydration Level.
Figure 6. The color chart for assessing hydration of the body from urine color. Your urine is a very good
indication of the exact nature of your internal body fluids. Examining it visually is always a good idea.
Drinks, other than Water, that Humans Consume in their Diets
Alcohol
Fermentation by yeast makes the ethanol contained in alcoholic beverages. The process of
fermentation is anaerobic, that is, it does not required Oxygen (O2). In ethanol fermentation,
simple sugars glucose, fructose, and sucrose are converted into cellular energy and produce
ethanol (C2H5OH) and carbon dioxide (CO2) as metabolic waste products. This liberation of CO2
is in fermentation is also what causes the rising of bread dough when prepared with yeast.
For Alcoholic Beverages, the fermentation process must occur in container or vessel that allows
CO2 to escape but prevents air, especially O2 from coming in. Exposure to O2 prevents ethanol
production and the generation of the gas formation of CO2 can buildup enormous pressure – and
actually be explosive. Think of when you shake a can of soda and then open it – that is the
release of high pressure CO2.
Coffee Naturally Contains Caffeine
Coffee and tea beverages that contain caffeine can act as diuretic too. It is worth knowing that
there is a difference between foods and beverages that naturally contain caffeine (like organic
coffee and tea and cocoa) and those that have synthetic caffeine added to them as a stimulant
(like coke, other sodas and monster drinks!). The un-natural sources are definitely worth
19
avoiding, in that they are not naturally compatible with the food/drink they are in and these
significant doses of caffeine will have a much more severe affect on your body.
Caffeine is a diuretic in a different way from alcohol, in that caffeine constricts certain vessels in
your kidneys (the efferent arterioles) and as a consequence, it produces more urine. Coffee is a
drink that naturally contains caffeine, and just as a point of interest, there are two basic varieties
of coffee plant: Arabica and Robusta.
The Arabica Coffee Plant
If on a coffee jar or tin there is statement that declares “100% Arabica”, then they are boasting
about their product. The Arabica coffee plant can only be grown at high altitudes in rich soils
and because of this it produces the very flavorful and aromatic coffee bean, with a nutty, mellow
and rich taste that is much sought after. The roasting of the bean will also affect its flavor, but if
you start with an Arabica bean, it is hard to go wrong! Robusta, on the other hand is the more
commonly sold coffee bean, and, as its name implies, it is robust and can grow many places. It
also naturally contains twice the amount of caffeine than Arabic beans and lacks that flavorful
taste. This is the coffee that most fast-food-like coffee joints use, as it is much cheaper and gives
a caffeine ‘punch’ that some have become accustomed to in association with drinking coffee.
Figure 7. Shown is a picture of perfectly ripe Arabica coffee beans on the plant.
What is Sweat?
Sweating, also known as perspiration, is the production of fluids secreted by the sweat glands in
the skin of mammals. The two types of sweat glands in humans are merocrine (much more
common) and apocrine glands (only in specific regions). Sweat is derived from blood.
Specifically, sweat is from the fluid portion of blood, plasma. Since plasma is 92% water, we can
guess that sweat is mostly water.
Sweating can be caused by normal physical exertion, fever, spicy foods, and high environmental
temperature. Strong emotions (anger, fear, anxiety etc.) and remembrance of past trauma can
also trigger profuse sweating. In humans, sweating is primarily a means of thermoregulation,
achieved by merocrine glands. Maximum sweat rates of an adult can be from 2 to 4 liters per
hour! Maybe even or 10 to 14 liters per day in adults! This all depends on what you are doing.
20
The evaporation of sweat from the skin surface has a cooling effect due to evaporative cooling.
As we know, the high heat of vaporization of water means that when water leaves the body in
vapor form, it take a lot of heat energy with it. Hence, in hot weather, or when the individual's
muscles heat up due to exertion, more sweat is produced. Animals with few sweat glands, such
as dogs, accomplish similar temperature regulation results by panting, which evaporates water
from the moist lining of the lungs, pharynx, oral cavity and nose.
Sweat also Contains:
Lactate, lysozyme, urea and minerals. The major minerals include sodium, chloride,
potassium, calcium, magnesium; plus trace elements such as zinc, copper, nickel, chromium,
iron, etc). The mineral composition varies with the individual, acclimatization to heat, exercise
level, the particular stress source that makes the sweat (sauna, etc.), the duration of sweating, and
the composition of minerals in the body.
Some exogenous organic compounds make their way into sweat as exemplified by an
unidentified odiferous "maple syrup" scented compound in several of the species in the
mushroom genus Lactarius. In humans, sweat is hypotonic or less concentrated with solutes than
plasma. This is mainly due to the lack of proteins in sweat.
Diaphoresis is the medical term for profuse sweating or perspiring; a diaphoretic is something
that has the power to cause increased perspiration.
Sweating most commonly occurs under the arms, on the feet, and on the palms of the hands.
There are from 2 to 4 million sweat glands throughout the body that will become completely
active during puberty. Although women tend to have more sweat glands, men's are generally
more active. When the body temperature rises, the autonomic nervous system stimulates the
eccrine glands to secrete fluid onto the surface of the skin, where it cools the body as it
evaporates.
The vast majority of sweat glands in the body are innervated by sympathetic "cholinergic"
neurons. Sympathetic postganglionic neurons usually secrete norepinephrine and are named
sympathetic adrenergic neurons. However, when sympathetic postganglionic neurons innervate
sweat glands they secrete acetylcholine and hence are termed sympathetic "cholinergic" neurons,
the only sympathetic postganglionic neurons known to secrete acetylcholine instead of
norepinephrine.
Pathological Causes of Diaphoresis
Diaphoresis may be associated with abnormal conditions, such as hyperthyroidism and shock. If
it is accompanied by unexplained weight loss or fever or by palpitations, shortness of breath, or
chest discomfort, it suggests serious illness.
Diaphoresis is also seen in an acute myocardial infarction (heart attack), from the increased
firing of the sympathetic nervous system, and is frequent in serotonin syndrome. Diaphoresis can
also be caused by many types of infections, often accompanied by fever and/or chills. Most
infections can cause some degree of diaphoresis and it is a very common symptom in some
21
serious infections such as malaria and tuberculosis. Diabetics relying on insulin shots or oral
medications may have low blood sugar (hypoglycemia), which can also cause diaphoresis.
Drugs (including caffeine, morphine, alcohol, and certain antipsychotics) may be causes, as well
as withdrawal from alcohol, benzodiazepines, non benzodiazepines or narcotic painkiller
dependencies.
The Sympathetic Nervous System stimulants such as cocaine and amphetamines have also been
associated with diaphoresis.
Mercury is well known for its use as a diaphoretic, used widely in the 19th and early 20th to
"purge" the body of an illness, but the high toxicity effects of mercury were initially erroneously
attributed to the disease being treated with mercurials.
Hyperhidrosis
In some, the body's mechanism for cooling itself is overactive, making them perhaps sweat four
or five times more than is necessary. Most commonly affects the armpits, feet, and hands, and
face, especially forehead. It is also possible to experience this condition over their whole body.
This condition can be embarrassing, as excessive uncontrollable sweating is obvious and often
unexpected. It is thought to be an inherited problem. A possible treatment modality is clipping of
the sympathetic nerve at the level of T4 by thoracoscopic means.
Anhidrosis or Hypohidrosis
The absence of sweat is actually a serious and life threatening problem. Anhidrosis
(hypohidrosis), is an insufficient ability to sweat. This leads to excess body heat and can causes
heat exhaustion. Sweat also excretes waste products and an inhibition of this elimination
mechanism is also deleterious. Symptoms include little or no perspiration, dizziness, flushing,
and muscle weakness. Unaffected areas may try to compensate by producing more perspiration,
so it's possible to sweat profusely on one part of the body and very little or not at all on another.