5.6 Reaction Rates Unit Planner
advertisement
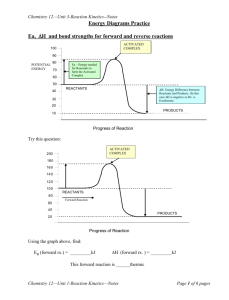
Riverside Campus MYP Unit Planner Unit Title: Teacher(s): Subject and grade level: Time frame and duration: Learning Profile Tick Box Reaction Rates Andrew Thomas, Zoe Badcock, Zoe Johnson Grade : 10 MYP: 5 3 weeks Learning Profile 1.CARING 2.OPEN-MINDED 4.PRINCIPLED 5.INQUIRERS 3.BALANCED 6.THINKERS Tick Box X Learning Profile 7.KNOWLEDGEABLE 8.COMMUNICATORS 9.REFLECTIVE Tick Box X Learning Profile Tick Box 10.RISK TAKERS 11.CREATIVE (Riverside Addition) 12.PROACTIVE (Riverside Addition) Significant Concept (Big Ideas) AOI Focus (Context) ATL (Skills) What factors affect the rate of reaction and what theory explains this? What happens to energy during reactions? How can humans use this to help them in day to day life and what are the consequences of this use? - decay of food – food preservatives - safety in industrial processes - petrol engines - creation of man made chemicals such as plastics and chlorofluorocarbons which do not biodegrade Community and Service Organisation Reflection Thinking Transfer Collaboration Communication Information Literacy Environments Human Ingenuity Health and Social Education ATL MYP Unit Question How have humans used their knowledge of chemistry to overcome or control the laws of nature and is this a good thing? Common Summative Assessment Task End of Unit Test Investigation – Factors affecting rates of Reaction Problem solving challenge – make a chemical clock Criteria assessed C D&E&F B&E&F Unit Overview Lesson Objectives 1. Introduce the guiding question and have students thinking about real life applications Learning Experiences, Teaching Strategies and Resources used. (Including Formative Assessment Task and Criterion links) http://www.youtube.com/watch?v=c0En-_BVbGc decomposition of fruit Use the images about rates of reaction images – divide class into groups, identify the picture, describe how it relates to rates of reaction, and have them come up with a story – relating to the guiding question and rates of reaction. See http://www.chemistryland.com/CHM107/EarlyChemistry/PreservationChemistry/PreservationChemistry.html for additional information. 2. Revisiting chemistry lab – review types of chemical reactions, writing equations and balancing them; - introducing s, l, g, aq to indicate states of reactants and products; know tests and methods of collection for gases. PowerPoint available with guiding question, significant concepts etc, and pictures Lab, “Writing and Predicting Products” When lab completed, go through each reaction and work out word then chemical equation. Introduce the symbols for states of reactants and products. Balance equations. Summarise the tests and methods for collecting gases – O2, CO2, H2, NH3 and Cl2 – (gas syringe and collection over water – not demonstrated - quantitative) – upward or downward delivery (qualitative), Extension and lead into Rates of Reaction – how can you speed up each reaction? 3. Factors affecting rate of reaction; graphing reactions, calculating initial rates of reaction; explaining graph with respect to reactants and products. Available worksheets... Revisiting Chemistry Intro Lab Revisiting Chemistry Extension Photocopy of p. 83 GCSE Chemistry on Gas Tests Write out equation... reactant 1 + reactant 2 product 1 + product 2 Ask students - decide what factors could affect how fast this reaction goes (reactant concentration – or pressure-; surface area of reactants; temperature; catalysts) - how the rate could be measured (measuring loss of mass; measuring gas produced; measuring formation of precipitate Draw typical graph of time vs. Concentration, first of products then of reagents. Explain what is happening to particles in reaction. (see fig 7.8, p.186 in Harwood, 2002, IGCSE Chemistry, Cambridge University Press) 4. Practical measurement of mass change to measure rate of reaction (surface area). Application of graphing skills. 5. Practical measurement of gas production to measure rate of reaction (concentration of reactant). Application of graphing skills. 6. Collision Theory 7. Investigation into factors affecting rates of reaction using CaCO3 & HCl Use graph to demonstrate calculation of initial rate of reaction (initial rate = rise at time t/time t) Experiment to show reaction of marble chips with dilute hydrochloric acid. Measure mass change through loss of CO2. Plot graphs and calculate initial rate of reaction. Change size of chips and compare. See lab sheet (?OMIT) Experiment to show reaction of magnesium ribbon and dilute hydrochloric acid. Measure gas produced using gas syringes and compare with displacement of water. Plot graphs and calculate initial rate of reactions. Change concentration of hydrochloric acid and compare. See lab sheet http://www.moletv.org.uk/watch.aspx?v=M52CB Summarise theory of reaction rates and collision theory – more collisions increases rate of reaction – higher temperature, concentration, larger surface area all increase number of collisions; catalysts increase number of successful collisions. See page 75 GCSE Chemistry Revision Guide, CGP Investigation assessing Planning (D), Data (E) & Attitudes (F) – LAB REPORT Students can change particle size, concentration of HCl, temperature They can measure gas produced using mass change, gas syringe or water displacement. Expect initial rate calculations and discussion of collision theory for top levels. 8. The Yellow Clock Challenge Application of rates of reaction to a problem See lab sheet Investigation assessing Communication (B), Data (E) & Attitudes (F) – LAB REPORT Students work in groups to plan and make a chemical clock – when reaction of sodium thiosulphate and hydrochloric acid produces enough yellow precipitate for a cross not to be visible anymore after exactly one minute. See lab sheet 9. Bonds contain energy and can be used to calculate energy change in a reaction Demo Endothermic Reaction - dissolving ammonium chloride in water Barium hydroxide + Ammonium Chloride Video – contains advanced terminology – entropy and moles (http://www.youtube.com/watch?v=5RJLvQXce4A&feature=player_embedded) It simply means that the bonding in the products is weaker than the reactants (the product is less stable than the reactants) and therefore requires energy from its surroundings to happen. Demo Exothermic Reaction – Mg + HCl or Na + H2O Draw graphs of progress of reaction vs Energy (kJ) (see p. 178-9 of Harwood; p. 78 of Revision Guide). Define the terms. Practice calculations of heat of reaction bond energy calculations 10. Measuring the energy in fuels See worksheets Experiment – comparing the energy content in three fuels and calculating the energy output per gram See pages181-183 of Harwood and p. 79 of CGP GCSE Chemistry Revision Guide 11. Reversible Reactions and Dynamic Equilibrium See lab sheet Demonstrate (or have students do this) – Heat hydrated copper sulphate, collecting water vapour in iced water bath – note change in colour; add the collected water back to CuSO4 and observe reverse reaction. This is an open system Demonstrate gentle heating of NH4Cl(s) NH3 + HCl in a closed test tube. This is a closed system. See worksheet 12. Review and Unit Test Hb + O2 is example of reversible reaction Assess Criterion C See Review Sheet and Test Differentiation Thinking Collaboration Content Links (Preceding or Succeeding Units) Cross-Curricula Links First unit in Chemistry but building on work done in Grade 9 with atomic structure, naming chemicals and writing balanced chemical equations. Also links to Biology unit on Enzymes in Grade 10. Approaches to Learning Reflection Teacher(s) Signature _____________________________ _____________________________ _____________________________ HOD Signature _________________________________