Synthesis and NMR and MS characterization of AJAY-4 {(E)-8-(2
advertisement
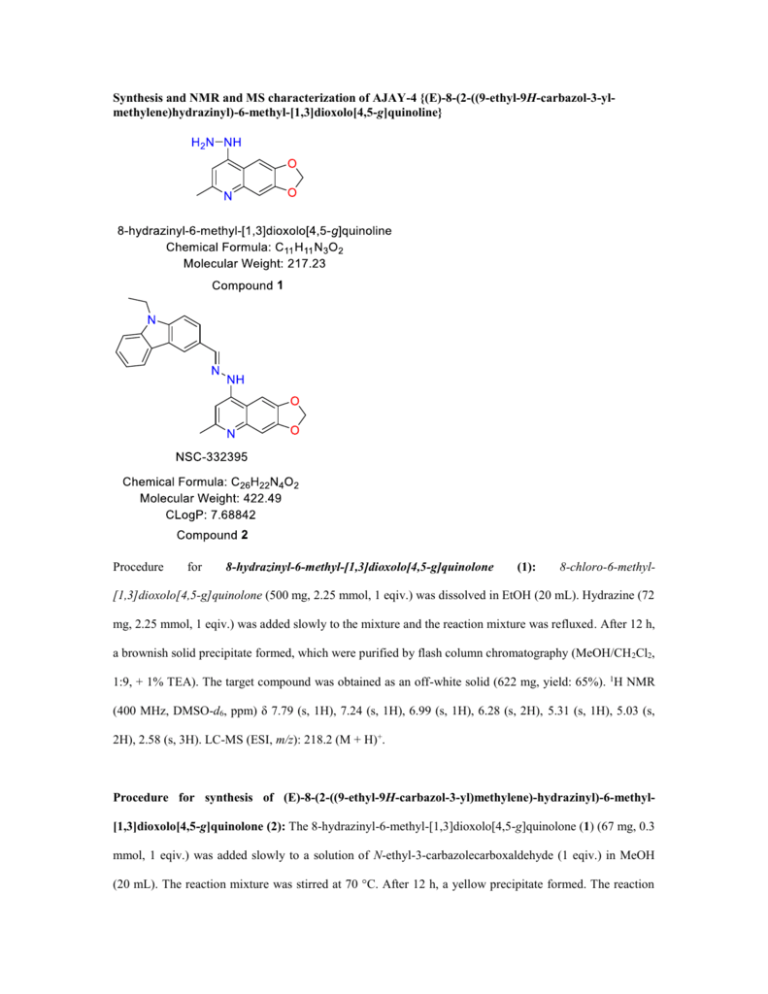
Synthesis and NMR and MS characterization of AJAY-4 {(E)-8-(2-((9-ethyl-9H-carbazol-3-ylmethylene)hydrazinyl)-6-methyl-[1,3]dioxolo[4,5-g]quinoline}
Procedure
for
8-hydrazinyl-6-methyl-[1,3]dioxolo[4,5-g]quinolone
(1):
8-chloro-6-methyl-
[1,3]dioxolo[4,5-g]quinolone (500 mg, 2.25 mmol, 1 eqiv.) was dissolved in EtOH (20 mL). Hydrazine (72
mg, 2.25 mmol, 1 eqiv.) was added slowly to the mixture and the reaction mixture was refluxed. After 12 h,
a brownish solid precipitate formed, which were purified by flash column chromatography (MeOH/CH 2Cl2,
1:9, + 1% TEA). The target compound was obtained as an off-white solid (622 mg, yield: 65%). 1H NMR
(400 MHz, DMSO-d6, ppm) δ 7.79 (s, 1H), 7.24 (s, 1H), 6.99 (s, 1H), 6.28 (s, 2H), 5.31 (s, 1H), 5.03 (s,
2H), 2.58 (s, 3H). LC-MS (ESI, m/z): 218.2 (M + H)+.
Procedure for synthesis of (E)-8-(2-((9-ethyl-9H-carbazol-3-yl)methylene)-hydrazinyl)-6-methyl[1,3]dioxolo[4,5-g]quinolone (2): The 8-hydrazinyl-6-methyl-[1,3]dioxolo[4,5-g]quinolone (1) (67 mg, 0.3
mmol, 1 eqiv.) was added slowly to a solution of N-ethyl-3-carbazolecarboxaldehyde (1 eqiv.) in MeOH
(20 mL). The reaction mixture was stirred at 70 °C. After 12 h, a yellow precipitate formed. The reaction
mixture was filtered and the precipitate washed with EtOH to obtain the target compound 2 as yellow solid
(58 mg, yield: 46%). 1H NMR (400 MHz, DMSO-d6, ppm) δ 8.3 (s, 1H), 8.66 (s, 1H), 8.32 (d, J = 8.0 Hz,
1H), 8.13 (s, 1H), 8.06 – 8.04 (m, 1H), 7.44 (d, J = 8.40 Hz, 1H), 7.69 (d, J = 8.40 Hz, 1H), 7.56 – 7.51 (m,
2H), 7.35 (s, 1H), 7.30 (t, J = 7.20 Hz, 1H), 6.36 (s, 2H), 4.55 – 4.49 (m, 2H), 2.73 (s, 3H), 1.37 (t, J = 7.20
Hz, 3H). LC-MS (ESI, m/z): 423.0 (M + H)+.








