Post-Secondary STEM Lesson Plan: Chemical Changes Chemical
advertisement
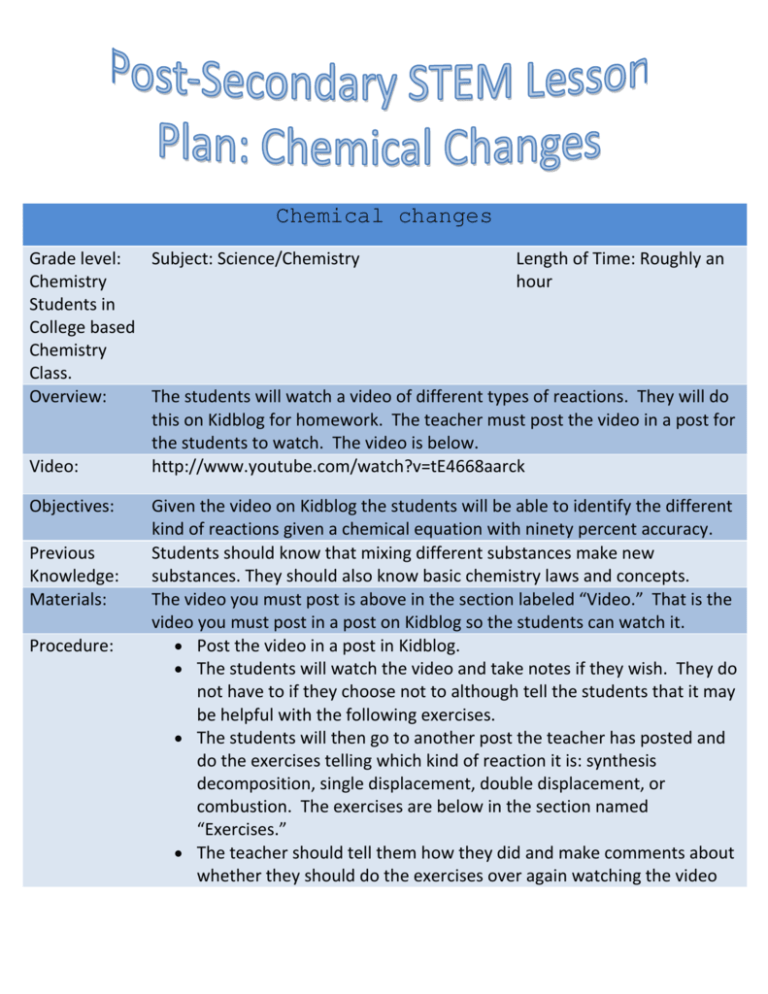
Chemical changes Grade level: Subject: Science/Chemistry Length of Time: Roughly an Chemistry hour Students in College based Chemistry Class. Overview: The students will watch a video of different types of reactions. They will do this on Kidblog for homework. The teacher must post the video in a post for the students to watch. The video is below. Video: http://www.youtube.com/watch?v=tE4668aarck Objectives: Previous Knowledge: Materials: Procedure: Given the video on Kidblog the students will be able to identify the different kind of reactions given a chemical equation with ninety percent accuracy. Students should know that mixing different substances make new substances. They should also know basic chemistry laws and concepts. The video you must post is above in the section labeled “Video.” That is the video you must post in a post on Kidblog so the students can watch it. Post the video in a post in Kidblog. The students will watch the video and take notes if they wish. They do not have to if they choose not to although tell the students that it may be helpful with the following exercises. The students will then go to another post the teacher has posted and do the exercises telling which kind of reaction it is: synthesis decomposition, single displacement, double displacement, or combustion. The exercises are below in the section named “Exercises.” The teacher should tell them how they did and make comments about whether they should do the exercises over again watching the video over again. Exercises: Grading: References: Indiana Academic Standards: A. 2K + Br2 -> 2KBr – synthesis B. Mg3N2 -> 3Mg + N2 –decomposition C. CH4 + 2O2 -> CO2 + 2H2O - combustion D. 4Al + 3O2 -> 2Al2O3 – synthesis E. Zn +CuCl2 -> ZnCl2 + Cu – single displacement F. 2KI -> 2K + I2 – decomposition G. Fe2O3 + 6HCl -> 2FeCl3 + 3H2O – double displacement H. H2 + Br2 -> 2HBr – synthesis I. 2C8H18 + 25O2 -> 16CO2 +2H2O - combustion J. Cl2 + 2NaBr -> 2NaCl + Br2 – single displacement K. Fe + Cl2 - > FeCl2 – synthesis L. 2NaCl -> 2Na + Cl2 – decomposition M. 2C6H6 + 15O2 -> 16CO2 +18H2O - combustion N. AgNO3 + HCl -> AgCl + HNO3 – double displacement O. Mg + 2HCl -> MgCl2 + H2 – single displacement P. 2N2O3 -> 2N2 + 3O2 - decomposition You should grade them on well they understood the material. If they did not do well have the repeat the exercise and watch the video over again. If they did well on the exercise then tell them they did a good job. http://www.youtube.com/watch?v=tE4668aarck http://www.eduref.org/Virtual/Lessons/Science/Chemistry/CHM0008.html http://dl.clackamas.edu/ch104-10/(10).htm http://dl.clackamas.edu/ch104-10/(11).htm http://dl.clackamas.edu/ch104-04/single.htm http://dl.clackamas.edu/ch104-04/double.htm http://library.thinkquest.org/11226/main/c18.htm Principles of Integrated Chemistry-Physics - CP.1 o Students begin to conceptualize the general architecture of the atom and the roles played by the main constituents of the atom in determining the properties of materials. They investigate, using such methods as laboratory work, the different properties of matter. They investigate the concepts of relative motion, the action/reaction principle, wave behavior, and the interaction of matter and energy.