Unit 3 Objectives - Hinsdale South High School
advertisement
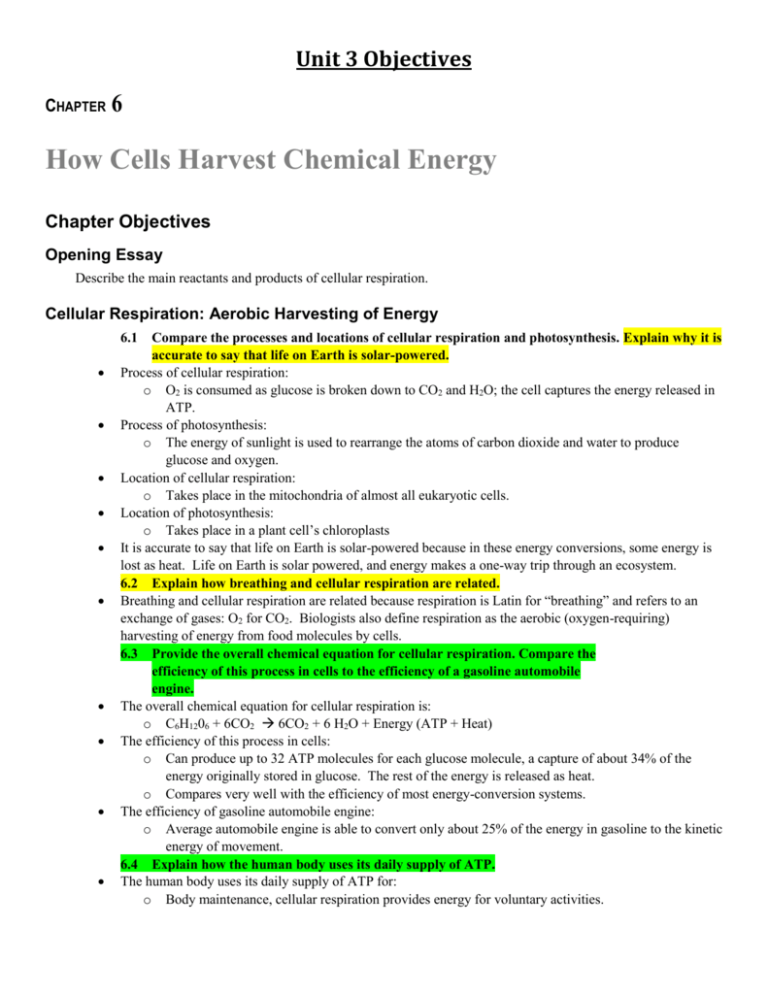
Unit 3 Objectives CHAPTER 6 How Cells Harvest Chemical Energy Chapter Objectives Opening Essay Describe the main reactants and products of cellular respiration. Cellular Respiration: Aerobic Harvesting of Energy 6.1 Compare the processes and locations of cellular respiration and photosynthesis. Explain why it is accurate to say that life on Earth is solar-powered. Process of cellular respiration: o O2 is consumed as glucose is broken down to CO2 and H2O; the cell captures the energy released in ATP. Process of photosynthesis: o The energy of sunlight is used to rearrange the atoms of carbon dioxide and water to produce glucose and oxygen. Location of cellular respiration: o Takes place in the mitochondria of almost all eukaryotic cells. Location of photosynthesis: o Takes place in a plant cell’s chloroplasts It is accurate to say that life on Earth is solar-powered because in these energy conversions, some energy is lost as heat. Life on Earth is solar powered, and energy makes a one-way trip through an ecosystem. 6.2 Explain how breathing and cellular respiration are related. Breathing and cellular respiration are related because respiration is Latin for “breathing” and refers to an exchange of gases: O2 for CO2. Biologists also define respiration as the aerobic (oxygen-requiring) harvesting of energy from food molecules by cells. 6.3 Provide the overall chemical equation for cellular respiration. Compare the efficiency of this process in cells to the efficiency of a gasoline automobile engine. The overall chemical equation for cellular respiration is: o C6H1206 + 6CO2 6CO2 + 6 H2O + Energy (ATP + Heat) The efficiency of this process in cells: o Can produce up to 32 ATP molecules for each glucose molecule, a capture of about 34% of the energy originally stored in glucose. The rest of the energy is released as heat. o Compares very well with the efficiency of most energy-conversion systems. The efficiency of gasoline automobile engine: o Average automobile engine is able to convert only about 25% of the energy in gasoline to the kinetic energy of movement. 6.4 Explain how the human body uses its daily supply of ATP. The human body uses its daily supply of ATP for: o Body maintenance, cellular respiration provides energy for voluntary activities. 6.5 Explain how the energy in a glucose molecule is released during cellular respiration. During cellular respiration, electrons are transferred from glucose to oxygen, releasing energy. Oxygen attracts electrons very strongly, and an electron loses potential energy when it “falls” to oxygen. 6.5 Explain how redox reactions are used in cellular respiration. They are used in cellular respiration by there being a loss of electrons from one substance which is called oxidation, and the addition of electrons to another substance is called reduction. A molecule is said to become oxidized when it loses one or more electrons and reduced when it gains one or more electrons. This is because an electron transfer requires a donor and an acceptor, oxidation and reduction always go together. Stripping electrons off of glucose, so it loses electrons, and oxygen takes electrons there by being reduced. 6.5 Describe the general roles of dehydrogenase, NADH, and the electron transport chain in cellular respiration. In the oxidation of an organic molecule, there are three carbons and a few of its other atoms. The general role of dehydrogenase, NADH, is to strip two hydrogen atoms from this molecule. The electron transport chain in cellular respiration undergoes a series of redox reactions in which electrons pass from carrier to carrier down to oxygen. The redox steps in the staircase release energy in amounts small enough to be used by the cell to make ATP. In reality, it’s the role of the electron carrier (dehydrogenase) but still look it up again. Stages of Cellular Respiration 6.6 List the cellular regions where glycolysis, the citric acid cycle, and oxidative phosphorylation occur. Note whether substrate-level phosphorylation or chemiosmosis occur at each of these sites. Glycolysis: o Cytoplasmic fluid of the cell. o Begins cellular respiration by breaking glucose into two molecules of a three-carbon compound called pyruvate. o Phosphorylation: a The Citric Acid Cycle: o Takes place within the mitochondria. o Oxidized to a two-carbon compound. o Next, completes the breakdown of glucose to carbon dioxide. o Main function of these first two stages, however, is to supply the third stage of respiration with electrons. o Phosphorylation: Oxidative Phosphorylation: o Requires an electron transport chain and a process known as chemiosmosis. o Chemiosmosis: the potential energy of this concentration gradient is used to make ATP. 6.7–6.12 Compare the reactants, products, and energy yield of the three stages of cellular respiration. First Stage: o Glycolysis: Reactants: Splitting of sugar Begins with a single molecule of glucose and concludes with two molecules of pyruvate (the ionized form of pyruvic acid). Glucose has six carbons, and these same six carbons end up in the two molecules of pyruvate (three carbons in each). There are nine chemical steps (look at Energy Yield) when going from glucose to pyruvate, each catalyzed by its own enzyme. As these reactions occur, two molecules of NAD+ are reduced to two molecules of NADH, and a net gain of two molecules of ATP is produced. Products: ATP is formed in glycolysis by the process called substrate-level phosphorylation. In this process, an enzyme transfers a phosphate group from a substrate molecule directly to ADP, forming ATP. Energy Yield: Energy extracted from glucose during glycolysis is banked in a combination of ATP and NADH. To use the energy in NADH, electrons from NADH must pass down an electron transport chain located in the inner mitochondrial membrane. Pyruvate molecules still hold most of the energy of glucose. Steps 1-4, the energy investment phase, actually consume energy. o ATP is used to energize a glucose molecule, which is then split into two small sugars that are now primed to release energy. Steps 5-9, the energy payoff phase, yield energy for the cell. o Two NADH molecules are produced for each initial glucose molecule, and four ATP molecules are generated. Second Stage: o The Citric Acid Cycle: Reactants: Pyruvate is transported from the cytoplasmic fluid, into a mitochondrion, where the citric acid cycle and oxidative phosphorylation will occur. Pyruvate doesn’t enter the citric acid cycle. Three Steps: o A carboxyl group is removed from pyruvate and given off as a molecule of CO2 (this is the first step in which CO2 is released during respiration. o The two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to NADH. o A compoiund called coenzyme A, derived from a B vitamin, joins with the two-carbon group to form a molecule called acetyl coenzyme A, abbreviated acetyl CoA. Products: Set up the second major stage of cellular respiration. Energy Yield: Each turn of the cycle makes one ATP molecule by substrate-level phosphorylation. Also produces four other energy-rich molecules: three NADH molecules and one molecule of another electron carrier, FADH2. Citric acid cycle processes two molecules of acetyl CoA for each initial glucose. Thus, two turns of the cycle occur, and the overall yield per molecule of glucose is 2 ATP, 6 NADH, and 2 FADH2. Third Stage: o Oxidative Phosphorylation: Reactants: For the cell to be able to harvest the energy banked in NADH and FADH2, these molecules must shuttle their high-energy electrons to an electron transport chain. There the energy from the oxidation of organic molecules is used to phosphorylate ADP to ATP- hence the name oxidative phosphorylation. Acetyl CoA enters Citric Acid Cycle CoA leaves Five steps next: o o Step one: Two carbons enter cycle and are combined with the four-carbon molecule oxaloacetate. The product of this reaction is the six-carbon molecule citrate. All the acid compounds in this cycle exist in the cell in their ionized for. Steps 2-3: Products: D Energy Yield: d Fermentation: Anaerobic Harvesting of Energy 6.13 Compare the reactants, products, and energy yield of alcohol and lactic acid fermentation. Distinguish between strict anaerobes and facultative anaerobes. Reactants of alcohol: o Recycle their NADH back to NAD+ while converting pyruvate to CO2 and ethanol. Reactants of lactic acid fermentation: o NADH is oxidized to NAD+ as pyruvate is reduced to lactate (the ionized form of lactic acid). o Muscle cells can switch to lactic acid fermentation when the need for ATP outpaces the delivery of O2 via the bloodstream. o The lactate is carried in the blood to the liver, where it is converted back to pyruvate and oxidized in the mitochondria of liver cells. Products of alcohol: o CO2 provides the bubbles in beer and champagne o Bubbles of CO2 generated by baker’s yeast cause bread dough to rise. o Ethanol is toxic to the organisms that produce it. o Yeasts release their alcohol wastes to their surroundings, where it usually diffuses away.. Products of lactic acid fermentation: o 2 Lactate Energy yield of alcohol: o Yield’s 2 ATP when Glucose is transformed to Pyruvate by adding 2 Phosphorus to 2 ADP. Energy yield of lactic acid fermentation: o Yield’s 2 ATP when Glucose is transformed to Pyruvate by adding 2 Phosphorus to 2 ADP. While strict anaerobes require anaerobic conditions and are poisoned by oxygen, can make ATP either by fermentation or by oxidative phosphorylation, depending on whether O2 is available. It happens outside the mitochondria because glycolysis happens in glycolysis. The whole idea of fermentation is to empty the electron carriers Muscle cells can go through lactic fermentation Connections Between Metabolic Pathways 6.15 Explain how carbohydrates, fats, and proteins are used as fuel for cellular respiration. Explain why a gram of fat yields more ATP than a gram of starch or protein. They are used as fuel for cellular respiration by: o Carbohydrates: Broken down into sugars which then hydrolyzed by enzymes into glucose which goes through the Glycolysis, then Citric Acid Cycle, and then Oxidative Phosphorylation. o Fats: Broken down into Glycerol or Fatty acids. Glycerol: o Gets converted into glyceraldehyde 3-phosphate (G3P), one of the intermediates in glycolysis. Fatty acids: o Go straight to the Citric Acid Cycle o Proteins: Broken into amino acids Lose the amino groups Goes to either pyruvate or Citric Acid Cycle 6.16 Explain how nutrients are used in biosynthesis. Amino Acids form proteins Fatty acids and glycerol form fats Sugars form carbohydrates All come out of the respiration cycle. CHAPTER 7 Photosynthesis: Using Light to Make Food Chapter Objectives Opening Essay Explain how plants can be used as a renewable energy source. Explain why this is better than burning fossil fuels. An Overview of Photosynthesis 7.1 Define autotrophs, heterotrophs, producers, and photoautotrophs. Autotrophs: o “self-feeders” in Greek o Make their own food and thus sustain themselves without consuming organic molecules derived from any other organisms. Heterotrophs: o Can’t make its own food molecules and must obtain them by consuming other organisms or their organic products. o A consumer or a decomposer in a food chain Producers: o Feed the consumers of the biosphere Photoautotrophs: o The ultimate source of organic molecules for almost all other organisms. o Often referred to as the producers of the biosphere because they produce its food supply 7.2 Describe the structure of chloroplasts and their location in a leaf. Identify specifically where most light energy is converted to chemical energy. Chlorophyll: o o Where the leaves green color comes from. A light-absorbing pigment in the chloroplasts that plays a central role in converting solar energy to chemical energy Mesophyll: o The green tissue in the interior of the leaf Stomata: o Tiny pores where carbon dioxide enters the leaf, and oxygen exits Structure: o An envelope of two membranes enclosing an inner compartment, which is filled with a thick fluid called stroma. o Inside the stroma is a system of interconnected membranous sacs, called thylakoids, which enclose another internal compartment, called the thylakoid space. o Thylakoids are concentrated in stacks called grana. o Inside of the thylakoid membranes are the chlorophyll molecules that capture light energy. o The thylakoid membranes also house much of the machinery that converts light energy to chemical energy, which is used in the stroma to make sugar. 7.3 Explain how plants produce oxygen. Describe the experiments that revealed the source of the oxygen produced during photosynthesis. Plants take in CO2 and H2O and produce sugar and oxygen When a plant was given CO2 containing 18O gave off no labeled (18O-containing) oxygen gas. When a plant given H2O containing 18O did produced labeled O2. 18 O is the isotope of oxygen These two experiments showed that the O2 produced during photosynthesis comes from water and not from CO2. 7.4 Describe the role of redox reactions in photosynthesis and cellular respiration. CO2 is reduced to sugar as electrons, along with hydrogen ions (H+) from water, are added to it. Cellular respiration: o Harvests energy stored in a glucose molecule by oxidizing the sugar and reducing O2 to H2O. o This process involves a number of energy-releasing redox reactions, with electrons losing potential energy as they “fall” down an electron transport chain to O2. o Along the way, the mitochondrion uses some of the energy to synthesize ATP. Photosynthesis: o Requires energy. o Potential energy of electrons increases as they move from H2O to CO2 during photosynthesis. o The light energy captured by chlorophyll molecules in the chloroplast provides this energy boost. o Converts light energy to chemical energy and stores it in the chemical bonds of sugar molecules, which can provide energy for later use or raw materials for biosynthesis. 7.5 Compare the reactants and products of the light reactions and the Calvin cycle. Explain how photosynthesis relates to these reactions. Reactants of the light reactions: o Light energy Chemical energy o Release O2. o Water is split, providing a source of electrons and giving off O2 as a by-product. Reactants of the Calvin Cycle: o Assemble sugar molecules using CO2 and the energy-rich products of the light reactions. o Carbon fixation: The incorporation of carbon from CO2 into organic compounds, shown in the figure as CO2 entering the Calvin Cycle. o After carbon fixation, enzymes of the cycle make sugars by further reducing the carbon compounds. Products of the Light reactions: o ATP from ADP and a phosphate group o Light energy absorbed by chlorophyll molecules built into the membranes is used to drive the transfer of electrons and H+ from the water to the electron acceptor NADP+ reducing it to NADPH. Products of the Calvin Cycle: o O2 and sugar Photosynthesis relates to these reactions because the first part of its name, Photo means light, and refers to the light reactions; synthesis, meaning “putting together,” refers to sugar construction by the Calvin cycle. The Light Reactions: Converting Solar Energy to Chemical Energy 7.6 Describe the properties and functions of the different photosynthetic pigments. Pigments: light-absorbing molecules Built into the thylakoid membranes. They absorb some wavelengths of light and reflect or transmit other wavelengths. We don’t see the absorbed wavelengths; their energy has been absorbed by pigment molecules. We see the green wavelengths that the pigments transmit and reflect. The absorbed energy moves the electrons to a more energetic orbital when chloroplast pigments absorbs light Chlorophyll a: o Participates directly in the light reactions, absorbs mainly blue-violet and red light. o Looks blue green because it reflects mainly green light. Chlorophyll b: o Similar to chlorophyll a. o Absorbs mainly blue and orange light and reflects (appears) yellow-green. o Broadens the range of light that a plant can use by conveying absorbed energy to chlorophyll a, which then puts the energy to work in the light reactions. Carotenoids: o Various shades of yellow and orange. o The cause for the leaves fall colors once the green chlorophyll breaks down. o May broaden the spectrum of colors that can drive photosynthesis. o However, a more important function seems to be photoprotection: Some carotenoids absorb and dissipate excessive light energy that would otherwise damage chlorophyll or interact with oxygen to form reactive oxidative molecules that can damage cell molecules. Similar carotenoids, which we obtain from carrots and some other plants, have a photoprotective role in our eyes. Each type of pigment absorbs certain wavelengths of light because it is able to absorb the specific amounts of energy in those photons. 7.7 Explain how photosystems capture solar energy. When a pigment molecule absorbs a photon of light, one of the pigment’s electrons jumps to an energy level farther from the nucleus. Light enters Photosystem II where it activates an electron from water. Next the electron goes through an electron transport chain to Photosystem I while creating Hydrogen ions which are released into the thylakoid space. Next, light energy hits Photosystem I where NADP + picks up the electron and transforms into NADPH. Finally, the ATP synthase picks up a Hydrogen ion from the thylakoid space and when it passes through the ATP synthase, it changes ADP + P into ATP. 7.8–7.9 Explain how the electron transport chain and chemiosmosis generate ATP, NADPH, and oxygen in the light reactions. A pigment molecule in a light-harvesting complex absorbs a photon of light. The energy is passed to other pigment molecules and finally to the reaction center of photosystem II, where it excites an electron of chlorophyll P680 to a higher energy state. o This electron is captured by the primary electron acceptor. o Water is split, and its electrons are supplied one by one to P680, each replacing an electron lost to the primary electron acceptor. The oxygen atom combines with a second oxygen from another split water molecule, forming O2. o Each photoexcited electron passes from photosystem II to photosystem I via an electron transport chain. The exergonic “fall” of electrons provides energy for the synthesis of ATP by pumping H+ across the membrane. o At the same time, in the reaction center of Photosystem I, light energy excites an electron of chlorophyll P700. o Photoexcited electrons of photosystem I are passed through a short electron transport chain to NADP+, reducing it to NADPH. The input of light energy boosts electrons in the reaction-center complexes of both photosystems up to the excited state. o The electrons are caught by the primary electron acceptor on to of the platform in each photosystem. o Photosystem II passes the electrons through an ATP mill. o Photosystem I hands its electrons off to reduce NADP+ to NADPH. o NADPH, ATP and O2 are the products of the light reactions. Look at the second and the third paragraphs of 7.9 if still confused. 7.9 Compare photophosphorylation and oxidative phosphorylation. Oxidative phosphorylation (ATP formation) o The high-energy electrons passed down the electron transport chain come from the oxidation of organic molecules. o Mitochondria transfer chemical energy from food to ATP. o The final electron acceptor is O2 o Electrons end up at a low energy level in H2O o Byproduct: water o E- acceptor: O2 o High-energy e-‘s come from oxidation of organic molecules o E- end up at low energy-level. Photophosphorylation: o Light energy is used to drive electrons that originally came from water to the top of the transport chain. o Chloroplasts transform light energy into the chemical energy of ATP (specifically the thylakoid membrane). o The final electron acceptor is NADP+ o Electrons are stored at a high state of potential energy in NADPH. o Byproduct: O2 o E- acceptor: NADP+ o High-energy e-‘s come from water at top of Electron Transport Chain and energy light is used to drive e-. o E-‘s DO NOT end up at low energy level in water but rather STORED at a high state of Energy potential in NADPH. The Calvin Cycle: Reducing CO2 to Sugar 7.10 Describe the reactants and products of the Calvin cycle. Explain why this cycle is dependent upon the light reactions. Reactants: o CO2 o ATP o NADPH Products: o G3P o o Steps: o o o ADP + P NADP+ In the carbon fixation step, the enzyme rubisco attaches CO2 to RuBP. A reduction reaction, NADPH reduces the organic acid 3-PGA to G3P using the energy of ATP. For every three CO2 molecules fixed, one G3P molecule leaves the cycle as product, and the remaining five G3P molecules are rearranged o Using energy from ATP to regenerate three molecules of RuBP. For one G3P molecule, the Calvin cycle consumes nine ATP and six NADPH molecules, which were provided by the light reactions. 7.11 Compare the mechanisms that C3, C4, and CAM plants use to obtain and use carbon dioxide. Note examples of plants that use each of these systems. C3: o Plants that use CO2 directly from the air, and carbon fixation occurs when the enzyme rubisco adds CO2 to RuBP. o Called C3 plants because the first product of carbon fixation is the three-carbon compound 3-PGA. EX: Soybeans; oats; wheat; and rice. C4: o CO2 enters mesophyll cell o Adaptation that provides o Fix carbon dioxide in their bundle-sheath cells, using a different enzyme than those used in C3 plants. This enzyme has a higher affinity of CO2 and will continue to fix CO2 even when concentrations are low o Has two separate cells that CAM: o Conserves water by opening its stomata and admitting CO2 only at night. o CO2 is fixed into a four-carbon compound, which banks CO2 at night and releases it during the day o Open stoma at night, to collect CO2, and closes at day and goes through Calvin cycle during the day. o Calvin cycle in the AM EX: Aloe and jade plants Photosynthesis Reviewed and Extended 7.12 Review the overall process of the light reactions and the Calvin cycle, noting the products, reactants, and locations of every major step. 7.11 When O2 builds up in a leaf, rubisco adds O2 instead of CO2 RuBP. A two-carbon product of this reation is then broken down in the cell. This process is called photorespiration because it occurs in the light and, like respiration, Oxidative Phosphorylation: Mitochondria Byproduct: H2O E- acceptor: O2 High-energy e-‘s come from oxidation of organic molecules E-‘s end up at low energy level Light Reaction ( Photosynthesis is an endergonic process in which electrons from water are transferred to carbon dioxide, reducing it to sugar. During the exergonic process of respiration, energy is released from sugar when the sugar is oxidized. Citrate is the first compound formed during the citric acid cycle. A number of intermediates can be siphoned off and used to generate amino acids. Glycolysis and the citric acid cycle both occur in the cytoplasm in bacterial cells. Because plants can make more organic material each day than they need to use as respiratory fuel and precursors for biosynthesis, they stockpile the extra sugar as starch, storing some in chloroplasts, as well as in roots, tubers, seeds, and fruits. Glycolysis occurs in the cytoplasmic fluid. The Calvin cycle uses carbon supplied by CO2 and energy and high-energy electrons supplied by ATP and NADPH, respectively, generated during the light reactions to assemble sugar molecules. The energy released by the electron transport chain is used to create a proton gradient across the inner mitochondrial membrane. Carbon monoxide blocks the transfer of electrons to oxygen. Eventually, no protons will be pumped across the membrane and no ATP is formed. The electron transport chain requires a membrane that will act as a barrier to hydrogen ions that are being pumped across this space. The only membrane in a bacterial cell is the plasma membrane. Fats are excellent fuels because they store so much energy for their mass. The majority of the energy in a molecule of glucose is lost as heat. Anaerobic: need an environment that has NO oxygen Aerobic: preferred because it needs ATP and there for oxygen.