Chemical Formula, Equation and reaction Review key
advertisement

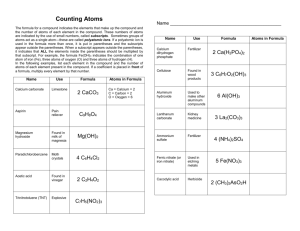
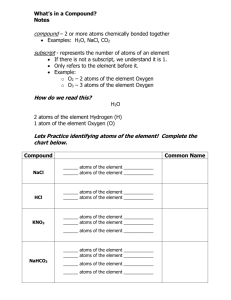
Name ______________________________ Chemical Formula, Equation, and Reaction Test Review Key 1. Write an example of a chemical formula. H2O 2. Write an example of a chemical equation. 2H2 + O2 2H2O 3. Describe the difference between a chemical formula and a chemical equation. Chemical formulas show a substance (compound) while chemical equations show a chemical reaction Counting Atoms 4. Use the following , 3Mg(OH)2, to answer the following: a. Identify an element Magnesium ( Oxygen or Hydrogen – also correct) b. Identify a compound 3Mg(OH)2 c. Number of total atoms 15 d. Number of molecules 3 e. Number of different elements f. Subscript g. Coefficient 3 2 3 h. Number of Magnesium atoms 3 i. Number of Oxygen atoms 6 j. Number of Hydrogen atoms 6 5. The starting materials in a chemical equation are found on which side of the equation? left What term is used to describe these materials? reactants 6. The substances created during a chemical reaction are found on which side of a chemical equation? Right What term is used to describe these materials? products 7. What separates the reactant and product in a chemical equation? Yield (arrow) 8. What is the first step used when balancing an equation? List and count atoms on each side of the equation 9. When balancing an equation, you should never change a subscript. You should only add a coefficient to the compound, therefore changing the number of atoms. 10. The law of conservation of mass tells us what about ….. the number of atoms on each side of an equation? Must be the same (equal) the materials found on each side of an equation? _ Must be the same (equal) the mass on each side of an equation? Must be the same (equal) Balance the following chemical equations. 11. 2 Na +____ I2 _2 NaI Na = ( 1) 2 I= 2 Na=(1) 2 I=(1) 2 12. 2 KClO3 _2_ KCl + _3 O2 13. ____ K3PO4 + _3_ HCl 3_ KCl + ____ H3PO4 14. ____ C3H8 + _5_ O2 _3_ CO2 + _4_ H2O 15. How does a chemical reaction affect the physical and chemical properties of the new substance that is formed? Get completely new properties 16. What factors can increase the rate of a chemical reaction? Temperature, concentration, surface area, and pressure 17. List 7 possible clues that indicate a chemical reaction has taken place. Bubbles, odor, precipitate, color change, produces light, produces electricity, temperature change 18. What happens to the bonds between atoms in a compound when a chemical reaction takes place?_break apart and re-form to create a new substance 19. How is a chemical change different from a physical change? Chemical change creates a new substance while physical change does not 20. Name 4 examples of a chemical change. Photosynthesis, Decomposition, cooking, burning 21. Name 4 examples of a physical change. Evaporation, condensation, decomposition, photosynthesis 22. What type of chemical reaction causes energy to be absorbed (decrease in temp.)? endothermic Identify one lab example the demonstrated this type of reaction. Citric Acid and Sodium Bicarbonate 23. What type of chemical reaction causes energy to be released (increase in temp.)? exothermic Identify one lab example the demonstrated this type of reaction. Rusting iron