5 THE PHOSPHORUS CYCLE

THE PHOSPHORUS CYCLE
I. Global Phosphorus pools
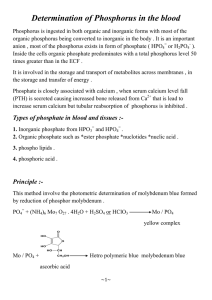
Most Phosphorus is stored in the crust as the mineral Apatite (Calcium
Phosphate -- Ca5(PO
4
)3), which is common in granites, but also occurs in metamorphic and sedimentary rocks. Apatite is found in teeth and bones, and sedimentary rocks containing these can also be a good source of Phosphate.
Some Phosphorus is also dissolved in fresh and ocean water, or is stored in soil or in organic matter.
II. Biological uses of Phosphorus
Nucleic Acids, which are made of nitrogen containing compound combined with a phosphate group and a sugar (N compound-CH
8
O
2
-PO
4
)
Adenosene di-Phosphate (ADP) and Adenosene tri-Phosphate (ATP), which is made of Adenine, a ribose sugar, and 2-3 Phosphate groups. These compounds are essential to life as they carry energy from mitochondria (who liberate energy in food) to the places in the cell where the energy is used
(muscles, metabolic processes, cell growth, etc.). Without these compounds, no life on Earth could live.
Redfield Ratios also consider the optimum ratio of Carbon to Phosphorus needed by life. Because the energy demands of land and water life is the same, the optimum ratio of C:P is 106 C:1 P for both.
Thus, the full Redfield Ratio (optimum ratio of C to N to P) for land and water life is:
Land: 106 C: 16 N: 1 P
Water: 106 C: 13 N: 1 P
We have already seen that N demands are greater in land life, as they need more proteins to make solid body structure. The other side of this is that as N
- demands are less in aquatic systems, the relative demand for P is higher, as
P is equally required by both land and water life. Thus, P usually limits growth of aquatic systems. This is why there is so much concern about limiting use of Phosphate detergents, as they will fertilize aquatic ecosystems, and allow some algae to take over.
III. The Phosphorus Cycle
Chemically, this is a much easier nutrient to deal with than N, as it only exists in one chemical state, Phosphate (PO
4
-3).
The only complex feature is that not all Phosphate molecules can readily dissolve. If the phosphate molecule does not dissolve, the phosphate con not be absorbed by living things.
Based on this, Phosphorus resources can be divided into 3 categories:
Free Dissolved P (Phosphate from inorganic molecules which have dissolved in water)
Dissolved Organic P (Phosphate from organic molecules which have dissolved in water)
Particulate P (Organic or inorganic Phosphate molecules which do no dissolve)
Factors which effect P levels in soil, water:
Geology (greater the amount of Phosphate minerals in rock, the greater the
P movement)
Soil Erosion Rates (greater rainfall levels, less vegetation creates higher rates of P movement)
Vertical Relief (greater the slope, the more erosion, and the greater the P movement)