1303adv_abridged
advertisement
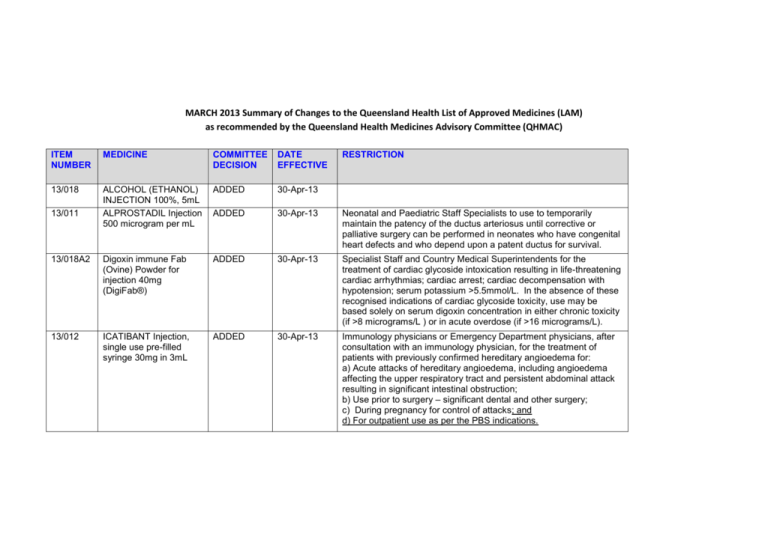
MARCH 2013 Summary of Changes to the Queensland Health List of Approved Medicines (LAM) as recommended by the Queensland Health Medicines Advisory Committee (QHMAC) ITEM NUMBER MEDICINE COMMITTEE DECISION DATE EFFECTIVE 13/018 ALCOHOL (ETHANOL) INJECTION 100%, 5mL ALPROSTADIL Injection 500 microgram per mL ADDED 30-Apr-13 ADDED 30-Apr-13 Neonatal and Paediatric Staff Specialists to use to temporarily maintain the patency of the ductus arteriosus until corrective or palliative surgery can be performed in neonates who have congenital heart defects and who depend upon a patent ductus for survival. 13/018A2 Digoxin immune Fab (Ovine) Powder for injection 40mg (DigiFab®) ADDED 30-Apr-13 Specialist Staff and Country Medical Superintendents for the treatment of cardiac glycoside intoxication resulting in life-threatening cardiac arrhythmias; cardiac arrest; cardiac decompensation with hypotension; serum potassium >5.5mmol/L. In the absence of these recognised indications of cardiac glycoside toxicity, use may be based solely on serum digoxin concentration in either chronic toxicity (if >8 micrograms/L ) or in acute overdose (if >16 micrograms/L). 13/012 ICATIBANT Injection, single use pre-filled syringe 30mg in 3mL ADDED 30-Apr-13 Immunology physicians or Emergency Department physicians, after consultation with an immunology physician, for the treatment of patients with previously confirmed hereditary angioedema for: a) Acute attacks of hereditary angioedema, including angioedema affecting the upper respiratory tract and persistent abdominal attack resulting in significant intestinal obstruction; b) Use prior to surgery – significant dental and other surgery; c) During pregnancy for control of attacks; and d) For outpatient use as per the PBS indications. 13/011 RESTRICTION ITEM NUMBER MEDICINE COMMITTEE DECISION DATE EFFECTIVE RESTRICTION 13/004 INDOCYANINE GREEN Injection, powder for reconstitution 25mg in 5mL ADDED 30-Apr-13 Surgical consultants, hepatologists and registrars for patients undergoing pre-operative assessment for liver resection. Where an item is not TGA approved, patients should be made fully aware of its status and appropriate consent obtained. 13/018 OSELTAMIVIR PHOSPHATE Powder for oral suspension 6mg/mL, 65mL ADDED 30-Apr-13 Specialist Staff and Country Medical Superintendents on the advice of an Infectious Diseases Physician or a Clinical Microbiologist or in accordance with an Infectious Diseases / Clinical Microbiology approved protocol for: a) Treatment of clinically suspected or laboratory proven influenza in inpatients only (not for general use in Emergency Departments unless admission is intended); and b) Prophylaxis of inpatients who are close contacts of inpatients with laboratory proven influenza on the advice of an infectious diseases physician, clinical microbiologist or infection control practitioner. Oseltamivir therapy should be ceased if influenza polymerase chain reaction (PCR) test of a nasopharyngeal swab or aspirate is negative. OR In accord with a QH pandemic influenza protocol. 13/013 MYCOPHENOLATE (SODIUM) Tablet 180mg, 360mg AMENDED 30-Apr-13 Specialist Staff for use in accord with Highly Specialised Drugs Program and PBS indications. 13/016 PREGABALIN CAPSULE 25mg, 75mg, 150mg, 300mg AMENDED 30-Apr-13 Specialist Staff and Country Medical Superintendents for use as per the PBS indications for neuropathic pain 13/005 GABAPENTIN CAPSULE 100mg, 300mg, 400mg AMENDED 30-Apr-13 13/014A RIVAROXABAN Tablet 10mg AMENDED 30-Apr-13 Specialist Staff and Country Medical Superintendents for partial epilepsy not controlled by other drugs; and continuation of existing patients being treated for neuropathic pain. (Note: In early 2014, gabapentin may no longer be LAM listed for neuropathic pain.)” For orthopaedic prophylaxis of venous thromboembolism in total hip and knee replacement. Page 2 of 6 ITEM NUMBER MEDICINE COMMITTEE DECISION DATE EFFECTIVE RESTRICTION 13/009 ROCURONIUM BROMIDE Injection 50mg in 5mL AMENDED 30-Apr-13 Emergency Specialists, Specialist Anaesthetists and Country Medical Superintendents 13/006 SUGAMMADEX SODIUM INJECTION 500mg in 5mL AMENDED 30-Apr-13 For use by credentialed anaesthetists and Emergency Physicians for emergency reversal of rocuronium in patients who cannot be intubated or cannot be oxygenated. [Only limited stock may be held outside pharmacy - on the difficult intubation trolley. All other use of sugammadex must be on an individual patient approval basis, with approval by the Director of Anaesthetics, the Director of Emergency Medicine or nominated representative, and in line with the SWAPNET guideline. (Available at http://qheps.health.qld.gov.au/patientflow/docs/swapnet_sugam_guid e.pdf)] 13/003 HYDROMORPHONE HYDROCHLORIDE Tablet, modified release 4mg, 8mg IV 5HT3 ANTAGONISTS FOR USE IN PONV (ONDANSETRON Injection 4mg in 2mL and GRANISETRON Injection 1mg in 1mL) LOSARTAN Tablet 25mg, 50mg DEFERRED 12/097 13/022 DEFERRED DEFERRED Page 3 of 6 ITEM NUMBER MEDICINE COMMITTEE DECISION 13/019 MAGNESIUM SULFATE in Water for Injection 4g/20mL (20%) and 10g/50mL (20%) Prefilled syringes DEFERRED 12/098 POSTOPERATIVE NAUSEA AND VOMITING (PONV) Guidelines SUCROSE Oral solution 24%, 1.5mL (Phebra) TRANEXAMIC ACID INJECTION 100mg/mL, 10mL DEFERRED 13/018 ALCOHOL (ETHANOL) INJ ABSOLUTE (5.5 Cal per mL) 20mL DELETED 30-Apr-13 13/018 BETAMETHASONE VALERATE CREAM 0.05%, 30g DELETED 30-Apr-13 13/018 BETAMETHASONE VALERATE OINTMENT 0.05%, 15g, 30g CIMETIDINE INJECTION 200mg in 2mL Digoxin immune Fab (Ovine) Powder for injection 38mg (Digibind®) DELETED 30-Apr-13 DELETED 30-Apr-13 DELETED 30-Apr-13 13/021 12/094 13/018 13/018A1 DATE EFFECTIVE RESTRICTION DEFERRED DEFERRED Page 4 of 6 ITEM NUMBER MEDICINE COMMITTEE DECISION DATE EFFECTIVE 13/018 DOCUSATE with GLYCERIN and SOFT SOAP Enema disposable 5mL DELETED 30-Apr-13 13/018 INDOMETHACIN EYE DROPS 10mg per mL (1%), 5mL DELETED 30-Apr-13 13/018 NICOTINAMIDE TABLET 250mg OSELTAMIVIR PHOSPHATE Powder for oral suspension 12mg/mL, 75mL DELETED 30-Apr-13 DELETED 30-Apr-13 13/018 PROCAINAMIDE HYDROCHLORIDE CAPSULE 250mg DELETED 30-Apr-13 13/018 PROCAINE HYDROCHLORIDE INJECTION 40mg in 2mL (2%) DELETED 30-Apr-13 13/018 STREPTOKINASE INJECTION 1,500,000 units SUGAMMADEX SODIUM INJECTION 200mg in 2mL DELETED 30-Apr-13 DELETED 30-Apr-13 13/020 OMEGA-3-ACID ethyl esters 90, Capsule 1,000mg NOT ADDED 13/008 BOTULINUM TOXIN TYPEAInjection100units NOT AMENDED 13/018 13/006 RESTRICTION Page 5 of 6 ITEM NUMBER MEDICINE COMMITTEE DECISION 13/014 FOR INFORMATION FINAL REPORT OF THE REVIEW OF ANTICOAGULATION THERAPIES IN ATRIAL FIBRILLATION NOTED DATE EFFECTIVE RESTRICTION Page 6 of 6