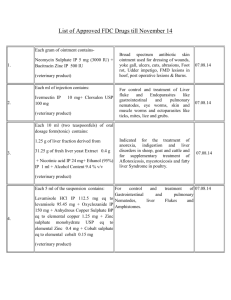
Pigs, broilers and turkeys: 8 g of «Gyramox
advertisement

ΠΑΡΑΡΤΗΜΑ 1: ΠΕΡΙΛΗΨΗ ΤΩΝ ΧΑΡΑΚΤΗΡΙΣΤΙΚΩΝ ΤΟΥ ΠΡΟΪΟΝΤΟΣ 1. Name of the veterinary medicine Gyramox, soluble powder for oral use for pigs, broilers and turkeys. 2. Qualitative and quantitative composition 100 g contain: Active principles Amoxicillin trihydrate 25.82 g (equal to amoxicillin 22.50 g) Flumequine 15.00 g Excipients For the complete list of the excipients see paragraph 6.1. 3. Pharmaceutical form Soluble powder for oral use to be dissolved exclusively in drinking water or liquid feed. 4. Clinical information 4.1. Target species Pigs, Broilers and Turkeys. 4.2. Indications for use specifying the target species The combination of amoxicillin and Flumequine is indicated for the three destination species in the treatment of bacterial diseases sustained by microorganisms that are sensitive to the two active principles, namely: Pigs: infections of the respiratory tract (bronchitis, bronchopneumonia, pneumonia, pleurisy, pulmonary disorders of viral infections); infections of the gastroenteric tract (enteritis, cholangiohepatitis); urinary infections (nephrite, cystitis); infections of skin and soft tissues (pedal infections and abscesses included); articular infections (polyarthritis); streptococcal infections; colibacillosis, salmonellosis and pasteurellosis. Broilers and turkeys. Salmonellosis; Colibacillosis; Staphylococcosis; Pasteurellosis; Secondary bacterial infections of viral infections such as C.R.D. 1/5 4.3. Contraindications Do not administer to animals that are hypersensitive to penicillins, to rabbits, small rodents, polygastric animals with functioning rumen, and to laying hens. Do not associate to treatments with systemic sulphonamides and Trimethoprim. 4.4. Special warnings for each target species Don’t administer to layers producing eggs for human consumption. 4.5. Special precautions Special precautions for the use in the animals Do not mix with solid feed. While using this veterinary medicine, always comply with the official and local regulations on the use of antimicrobial drugs. If possible, the quinolones should be used only based on the results of the antibiotic assay. A use of such products not compliant with the instructions supplied in the SPC may lead to an increase in the prevalence of bacteria being resistant to the quinolones and also reduce at the same time the efficacy of the treatment with other (fluoro)quinolones because of cross-resistance. Special precautions to be taken by the person administering the product to the animals During handling contact with the product must be avoided. For this aim it is a good practice wear suitable protective clothes. Avoid wasting the environment with the product. For handling product utilise suitable protective gloves. In case of accidental contamination, wash accurately with water and soap. Persons with known oversensitivity towards the product must avoid the contact with it. 4.6. Adverse reactions (frequency and seriousness) Gastrointestinal troubles and oversensitivity reactions that vary from light urticaria to lethal anaphylactic shock. Can occurs cross allergy with all other penicillins. Should any serious adverse reactions or other not mentioned in this label reaction occur, please inform the responsible veterinarian. . 4.7. Use during pregnancy, lactation or laying Utilise only in accordance to the evaluation of the risk/benefit ratio of the responsible veterinarian. 4.8. Interactions with other medicines and further forms of interaction The simultaneous administration of amoxicillin with bacteriostatic antibiotics, such as tetracyclines and macrolides, should be avoided as the antibacterial activity of amoxicillin is reduced. Synergism of action occurs when amoxicillin is administered along with cloxacillin, polimixins (such as colistin) and aminoglycosides (such as streptomycin, neomycin, gentamycin and kanamycin). Cross-resistance with penicillins develops rapidly, especially with ampicillin. The combination with clavulanic acid causes enhanced action. The administration of flumequine is not compatible with that of systemic sulphonamides and trimethroprim. 2/5 4.9. Dosage and way of administration Pigs, broilers and turkeys: 8 g of «Gyramox » every 100 kg of body weight (equal to 18 mg of amoxicillin and 12 mg of flumequine per kg of body weight) for 5 days. The product should be administered in drinking water or liquid feed according to the instructions of the prescribing Veterinarian, taking care not to exceed the daily dose of active principle allowed (expressed in mg/kg of body weight). The concentration in drinking water should be calculated according to the body weight of the animals to be treated and the water consumption. It is recommended to give no water to animals in the hours immediately prior to the treatment. 4.10. Excessive doses (symptoms, emergency procedures, and antidotes) Not available data. Do not exceed the recommended doses. 4.11. Withdrawal time Pigs: Broilers: Turkeys 5. 5 days 3 days 3 days Pharmacological properties ATC vet code: QJ01CA04 amoxicillin QJ01MB07 flumequine Pharmacotherapeutic group: anti-infective for systemic use. 5.1 Pharmacodynamics properties Amoxicillin is a semisynthetic penicillin with bactericidal activity against many Gram-positive and Gram-negative bacteria. Particularly sensitive microorganisms: Streptococci and Staphylococci not producing penicillinase, Pasteurella spp., Haemophilus spp., Bacillus anthracis, Bacillus subtilis, Brucella spp., Clostridium spp., Corynebacterium spp., Erysipelothrix rhusiopathiae, Fusiformis spp., Listeria monocytogenes and Spherophorus necrophorus. Moderately sensitive microorganisms: Salmonella spp., Streptococcus faecalis, Treponema hyodysenteriae, Moraxella spp., Escherichia coli, Proteus mirabilis and Vibrio cholerae. Amoxicillin acts by inhibiting the synthesis of peptidoglycan, which forms the wall of the bacterial cell. Amoxicillin performs a stronger bactericidal activity than ampicillin, which means a better therapeutic effect. Flumequine is a quinolone of synthesis which is mainly active against Gramnegative bacteria. The following bacteria are sensitive to its action in general: Aeromonas, E. coli, Klebsiella, Enterobacter, Yersinia, Salmonella, Pasteurella, Proteus, Vibrio anguillarum. It performs its bactericidal activity by inhibiting the replication of bacterial DNA. It is active when exudates, organic liquids, plasma proteins and serum are present. Amoxicillin and flumequine, by performing their bactericidal activity at various levels, prevent the onset of cross-resistance phenomena and the selection of resistant bacterial strains. The synergic action of the two active principles 3/5 assures a better activity mainly against those Gram-negative microorganisms that are less sensitive to amoxicillin. 5.2 Pharmacodynamics information Oral amoxicillin is absorbed more rapidly and better than ampicillin, due to its high resistance to the gastric secretions' acidity. Compared to the dose given orally, the share of amoxicillin actually absorbed by the gastrointestinal tract is around 70%, thus reaching high levels in blood. The absorption is not influenced in any way by the simultaneous administration of foods. Thanks to its high fat solubility, amoxicillin spreads evenly in all the body's tissues. If no inflammatory events involving the meninges are present, amoxicillin passes through the blood-brain barrier scarcely. The antibiotic is eliminated in its active form mainly through the urinary tract, just a small part of it is metabolized by liver into penicilloic acid. 80% of the medicine which is eliminated through the urinary tract is excreted through tubular secretions, the remaining 20% by glomerular filtration. Amoxicillin is an extremely safe antibiotic, as it is well tolerated by the animals. The flumequine is a quinolone of synthesis, active mainly against Gramnegative bacteria. To its action in general are susceptible bacteria: Aeromonas, E. coli, Klebsiella, Enterobacter, Yersinia, Salmonella, Pasteurella, Proteus, Vibrio anguillarum. Exerts its bactericidal action by inhibiting bacterial DNA replication. It is active in the presence of exudates, body fluids, plasma and serum proteins. 6. 6.1. 6.2. Pharmaceutical information List of excipients Sodium carbonate Anhydrous glucose Incompatibilities See contraindications and interactions. Lacking compatibility studies don’t mix with other veterinary medicines. 6.3. Shelf life Shelf life of the veterinary medicine packaged for selling: 24 months. Shelf life after the first opening of the primary package: 6 months. Shelf life after dilution or reconstitution in accordance with instructions: 12 hours 6.4. Special storage precautions The product must be stored in a dry place, at a temperature lower than +25 °C. Keep out of children’s reach. 6.5. Nature and contents of containers The product is packaged in 100 g packet, 1 and 5 kg bags of polyethylene aluminium – polyester (PET/AL/PE). 6.6. Special precautions to be taken for the disposal of unused veterinary medicines and/or waste materials All the unused veterinary medicines or the waste derived from these medicines must be disposed in compliance with local laws. 4/5 7. Holder of marketing authorisation Vétoquinol Italia S.r.l., Via Piana 265 – 47032 Bertinoro (FC) 8. Marketing authorisation numbers CY 00054V 9. Date of first marketing authorisation or authorisation renewal Date of first authorisation: 30/4/2004 Date of renewal: 2/11/2012 9. Date of text renewal: 15/01/2014 5/5