A risk assessment of MERS-CoV compared to SARS-CoV
advertisement

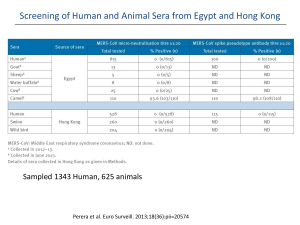
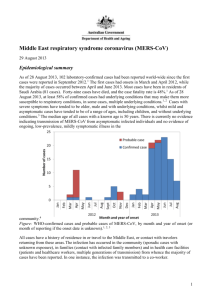
A risk assessment of MERS-CoV compared to SARS-CoV A risk assessment of MERS-CoV compared to SARS-CoV Maartje van Geenen (9756000) Supervised by Dr. B.J. Bosch; second reviewer: Dr. R.J. de Groot Abstract This century two novel coronaviruses appeared within a decade, causing both severe respiratory disease in humans. The severe acute respiratory syndrome corona virus (SARS-CoV), which originated in China in November 2002, was the first coronavirus to cause a pandemic in humans through zoonotic transmission with a 10% case fatality rate, causing approximately 8000 victims worldwide. Even though SARS-CoV transmission was contained within a year, fear remained that other coronaviruses in zoonotic reservoirs might also cross the barrier to humans and evolve human to human transmission like SARS-CoV did. This fear became reality in September 2012, when the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was discovered in Saudi Arabia, causing severe lower respiratory symptoms in humans with an estimated case fatality rate of 40%. Because of the phylogenetic relatedness of both viruses and similarities in clinical symptoms and pathology, a risk assessment of MERS-CoV compared to SARS-CoV is made, based on the knowledge available for both viruses thus far. This way, a better preparedness for new outbreaks of MERS-CoV and SARS-CoV can be established and the risk of a new pandemic with the newly discovered MERS-CoV can be assessed. Introduction It has long been known that viruses can cross species barriers and adapt from animals to humans to sometimes evolve human to human transmission, as was seen for small pox, measles and influenza. The latter, also called the Spanish flu, caused a pandemic back in 1918-19, killing approximately 50 million people. More recent examples of viruses crossing species barriers are H5N1 and H7N9 influenza, which crossed-over from poultry to humans with cases reported in Asia, even though no efficient human to human transmission was reported for both viruses thus far. A virus which did evolve human to human transmission was SARS-CoV, which crossed over from bats via intermediate hosts to humans in China. SARS-CoV was the first coronavirus causing a pandemic back in 2002/2003, after which a search began for novel coronaviruses with human to human transmission like SARSCoV1,2. In September 2012 the 6th coronavirus was discovered with human to human transmission, but it was the second coronavirus, after SARS-CoV, which caused severe symptoms of the lower respiratory tract, and therefore deserved extra attention in fear of the next coronavirus causing a global epidemic3. The new coronavirus, formerly known as human coronavirus-Erasmus Medical Center (EMC/2012)4,is now officially named Middle East Respiratory Syndrome Coronavirus (MERSCoV)5, because all index patients appear to originate from the Middle-East, in contrast to the Asian origin of SARS-CoV. Like SARS-CoV, MERS-CoV seems to be originating from coronaviruses circulating in bats6-9.In the case of SARS-CoV, intermediate hosts were identified, namely Himalayan palm civets (Paguma larvata) and raccoon dogs (Nyctereutes procyonoides) present on the Chinese wet markets, establishing a reservoir for the virus from which it could adapt to humans10-12. For MERS-CoV such a zoonotic reservoir has not yet been clearly established, despite the recent isolation of the first MERSCoV from a camel on a Qatari farm, with fragment analysis showing sequence alignment to two confirmed MERS cases who had reported contact with the camels on that farm13. Also the presence of neutralizing antibodies in dromedary camels from Qatar, Oman and Egypt support the idea that camels are the intermediate reservoir of MERS-CoV 13,14,15. Despite the similar clinical symptoms and similar phylogenetic background7, the viruses use different human receptors to enter the cells. SARSCoV uses the angiotensin-converting enzyme 2 (ACE2) as a receptor, while MERS-CoV uses dipeptidyl 1 A risk assessment of MERS-CoV compared to SARS-CoV peptidase 4 (DPP4), also known as CD26, as a receptor to enter the cells16-19.The difference in the receptor usage establishes a small difference in human cellular tropism. MERS-CoV has a preference of infecting unciliated bronchial epithelial cells, while SARS-CoV primarily infects ciliated bronchial epithelial cells20. Thus far, only limited human to human transmission has been documented for MERS-CoV in contrast to SARS-CoV back in 2003. The case fatality rate of MERS-CoV, however, seems to exceed the one of SARS-CoV fourfold (40% compared to 10%)21,22, which might be an overestimation with presumable undetected cases. If MERS-CoV were to adapt more efficiently to humans, as SARS-CoV appears to have done, an epidemic with a mortality rate exceeding that of SARS could be expected when no proper precautions will be taken. Whether that assumption is ungrounded fear or a risk that should be taken into consideration, will be investigated here. A risk assessment of MERS-CoV compared to SARS-CoV will be made, based upon available epidemiological data, human cellular tropism, transmission and pathogenesis, and immune evasion strategies and possible therapeutics. This way, better insights for dealing with new outbreaks of SARS-CoV and MERS-CoV can be established. First, some general aspects of coronavirus structure and replication will be discussed. Coronavirus: structure and replication Coronaviruses are enveloped, positive, single stranded RNA viruses with a large genome of about 30 kB. They belong to the family Coronaviridae, subfamily Coronavirinae, and are subdivided again into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus based upon genetic analyses5. The genus Betacoronavirus is subdivided into four lineages; A, B, C and D. SARS-CoV and MERS-CoV both belong to the genus Betacoronavirus, but SARS-CoV is classified into lineage B and MERS-CoV into lineage C5. This implies that both viruses have a relative close phylogenetic relationship with a common ancestor not that long ago (figure 1). Coronavirus particles enter permissive host cells by the attachment of the N-terminal S1 domain of their spike protein (S protein) to the cellular receptor, followed by endocytosis. SARS-CoV uses the receptor ACE2, while MERS-CoV uses the recently identified receptor DPP416,17. Membrane fusion of the envelope of the virion with the cell membrane or endocytotic vesicle is mediated by the Cterminal transmembrane S2 domain of the S protein. Coronavirus S proteins resemble class I fusion proteins in which cleavage between the S1 and S2 domain occurs by host cell proteases, activating the S protein for fusion23, even though cleavage is not essential for infection and some coronaviruses are not cleaved at all. It has been determined that host cell proteases are also necessary for activation of the S protein of SARS-CoV24,25. Amongst others, Cathepsin L in endosomal compartments, the transmembrane serine protease TMPRSS2 and the human airway trypsin-like protease (HAT), which was found co-expressed with ACE2 on human lung epithelium, have been proposed as activating cellular proteases for SARS S26-28. MERS-CoV also requires activation by cellular proteases, as cathepsin L and B in endosomes and the transmembrane serine protease TMPRSS2 have been proposed as two distinct activation pathways29,30. After viral entry and uncoating, replication of coronavirus particles takes place in so far determined Endoplasmic Reticulum (ER) derived vesicles in the cytoplasm of infected cells1,18. The viral genome is replicated through a full negative sense intermediate, in single or double membrane vesicles by the virally encoded replicase proteins. These replicase proteins are directly transcribed from the full length positive sense RNA genome and are encoded in the first of the at least 6 open reading frames (ORFs) in the coronavirus genome called ORF1a/b (figure 2)1,18. ORF1a produces the polypeptide pp1a and when translation is continued after ORF1a into ORF1b after a –1 ribosomal frame shift, it produces pp1ab. Both polypeptides are processed by virally encoded proteases. After proteolitic processing, 15 or 16 non-structural proteins (nsps) are generated, which are involved in replication or virus-host interactions1. 2 A risk assessment of MERS-CoV compared to SARS-CoV Figure 1 Phylogenetic tree of Coronavirinae subfamily. MERS-CoV belongs to lineage C of genus Betacoronavirus, while SARS-CoV belongs to lineage B of the same genus, indicating that both viruses having a relative close phylogenetic relationship, based upon genetic analyses5. The 3’ one-third of the genome codes for the structural proteins as well as possible accessory proteins, as is the case for SARS-CoV and MERS-CoV. The structural proteins consist of the S protein, which project as trimers on the outer surface, the membrane glycoprotein (M), the envelope protein (E) and the nucleocapsid protein (N) (figure 3)18. The accessory genes, interspersed between the structural genes (figure 2), code for proteins which improve replication in naturally infected cells, even though they are generally dispensable for replication in vitro1. In lineage A Betacoronavirus an additional accessory protein is expressed; the hemagglutinin-esterase (HE) which binds reversible to O-acetylated sialic acids, due to the lectin (mediating binding) and sialate-O-acetylesterase (mediating release) properties of HE31,1,18. Figure 2 Example of coronavirus genome organization. The coronavirus SARS genome is used as an example to show coronavirus genome organization. The first ORF (ORF1a and 1b) at the terminal 5’ end comprises the replicase gene and is translated into polyproteins pp1a and, after a -1 ribosomal frame shift, to pp1ab. Proteolitic processing of the polyproteins yields 16 nonstructural proteins (nsps) for replication. The rest of the coronavirus two thirds of the genome consists of the four structural genes (in blue) with 8 interspersed accessory genes (in red)18. 3 A risk assessment of MERS-CoV compared to SARS-CoV Figure 3 Schematic representation of a coronavirus. The four common structural proteins of the coronavirus virion are indicated: the spike protein (S), the membrane protein (M), the envelope protein (E) and the nucleocapsid protein (N)18. The structural and accessory proteins are produced from subgenomic mRNAs, which are not directly derived from the full length negative sense RNA genome, but are generated through a similar set of subgenomic minus sense RNA templates. All coronavirus mRNAs have a common leader sequence, encoded at the 5’ end of the genome. All subgenomic mRNAs start from the 3’ end of the genome until they encounter a transcription regulatory sequence (TRS) of an ORF, which can be thousands of nucleotides apart from the common 5’ leader sequence. After the encounter of the TRS, the transcript is transferred to the 5’ side of the genome where it basepairs with the TRS residing in the 5’ positive sense genome. From here, the leader sequence residing upstream, is copied. This way, each negative sense subgenomic RNA becomes linked to the common 5’ leader sequence through a process called discontinuous 3’ extension1,18. The subgenomic negative sense RNAs then serve as a template for subgenomic mRNA production(figure 4)18. Figure 4 Coronavirus genome replication. The full length positive strand genome is replicated through a full length negative strand intermediate (a) from which the replicase gene is directly translated. The structural and accessory genes are translated from subgenomic mRNAs, which are transcribed from negative sense subgenomic RNAs (e). These latter RNAs arise from discontinuous 3’end extension (b,c,d), where transcription starts from the 3’end until a TRS is met (b). The transcript is then transferred to the 5’end (c) where the transcribed TRS base pairs with the genomic TRS Clinical symptoms of MERS- and SARS-CoV and the common leader sequence at the 5’ end of all mRNAs can be transcribed18. 4 A risk assessment of MERS-CoV compared to SARS-CoV SARS-CoV infection and MERS-CoV infection are both characterized by lower respiratory tract disease, manifesting in mild to severe pneumonia which can occasionally develop into acute respiratory distress syndrome (ARDS) and/or renal failure32,33. Both coronavirus infections have also reported gastrointestinal symptoms such as diarrhoea in about one third of the cases, with gastrointestinal shedding occurring in 70-100% of the cases for SARS. In the case of SARS-CoV infection, systemic spread was also reported18, which has not yet been confirmed for MERS-CoV. The median incubation time for SARS-CoV is 4 to 6 days with initial symptoms starting within 2 to 10 days after exposure19. The incubation time for MERS-CoV is estimated generally at less than one week, even though it might extend in a minority of cases up to 9-12days3,34. So despite some minor differences, SARS- and MERS-CoV clinically present itself very similarly. To assess whether MERS- and SARS-CoV infection can be classified into the same risk category, other aspects need to be compared as well, such as available epidemiological data. Epidemiology of MERS- and SARS-CoV Epidemiological data, such as incidence, prevalence and mortality rate, provide tools for risk assessment of viral infections. Since April 2012 to November 2013, 176 MERS-CoV cases, including 19 non-laboratory confirmed cases, have been reported to the WHO, with a mortality rate of 40%22. In order to assess the pandemic risk of MERS-CoV compared to prepandemic SARS-CoV, one can calculate the basic reproduction number (R0) to evaluate the transmissibility of the virus. R0 represents the number of patients with secondary infections per patient with a primary infection, in a fully susceptible environment. A R0 above 1 means that epidemic potential has been reached. In order to calculate R0, cases need to be properly registered, after which clusters can be formed based upon temporal and geographical information. The clusters can again be subdivided into transmission trees after investigation of relationships and contacts of patients, taking into account the minimal and maximal incubation time of the infection. The R0 of MERS-CoV was calculated at the end of June 2013 at 0.6 in an optimistic case scenario and at 0.69 in the worst case scenario, the latter assuming a small number of large transmission trees35. Recently, phylogenetic analysis of MERS-CoV genomes of 21 new and confirmed clinical samples, in addition to nine published MERS-CoV genomes, support the optimistic case scenario R0 of 0.636. Cotton et al., 2013, found genetic diversity of the MERS-CoV genomes in the Riyadh cluster in SaudiArabia, supporting the idea of multiple virus introductions from a geographically disperse MERS-CoV zoonotic reservoir, or alternatively, supporting human-to-human transmission from infected people who are continuously moving. However, the R0 can be different under various circumstances. Approximately 26% of all MERS cases is acquired in health care settings37. The R0 of MERS-CoV in hospitals can increase up to epidemic potential with even 1 patient infecting 7 other persons, as was thought to have occurred in the nosocomial outbreak of MERS in 4 healthcare facilities in Al-Hasa, Eastern Saudi Arabia38. Recently, Cotton et al., 2013, also found genetic diversity in the Al-Hasa cluster of 23 confirmed cases, suggesting that this hospital outbreak of MERS might also consist of multiple virus introductions, decreasing the assumed high R0 of MERS in health care settings36. The overall calculated R0 of MERS-CoV of 0.6 might be an underestimation of the actual R0, if transmission of MERS-CoV were to also occur via asymptomatic or mildly symptomatic people. In support of this, calculations based upon non-resident cases returning home from the Middle East suggest that 62% of MERS cases have been missed up to Aug 8, 201339. It must be stated though that for those calculations it was assumed that visitors and residents are at equal risk of MERS-CoV infection. This is not necessarily the case if visitors are more likely to be exposed to the actual source 5 A risk assessment of MERS-CoV compared to SARS-CoV by means of e.g. camel rides. Furthermore, proper control measures in areas of MERS can also give an underestimation of the actual R0 of MERS-CoV. If no control measures were present, the R0 could increase up to epidemic potential (0.8-1.3), as was calculated by Couchemez et. al, 201439. So, despite the evidence so far of a large number of small transmission trees for MERS-CoV36, supporting an optimistic R035, the R0 is dependent on the setting in which MERS occurs and on the total number of actual MERS cases, the latter not yet being clearly established. Therefore, ruling out epidemic potential for MERS-CoV at this moment in time, might be too early. Comparing the epidemiological data of MERS-CoV with SARS-CoV, 8098 confirmed and probable cases were reported with a 10% mortality rate (774 deaths) from November 2002 to July 2003 19,21. The R0 of prepandemic SARS was estimated at 0.835, with each SARS case infecting approximately 2-4 people40. The incidence and the prepandemic R0 of SARS-CoV greatly exceeds that of MERS-CoV at this moment, supportive of the idea that MERS-CoV to this day does not form a pandemic threat. In health care settings, the R0 of SARS-CoV, like MERS-CoV, could increase far above 2-341-43. The SARS epidemic of 2003 was characterized by events of ‘’superspreading’’ in dense settings such as hospitals, defined as 1 primary case causing an above average number of secondary cases44. Various nosocomial outbreaks of SARS were documented in Asia and Canada as reviewed by Cheng et al., 201345, the earliest nosocomial outbreak of SARS occurring in Guangzhou, China, with 1 patient causing over 80 secondary cases43. Even though MERS and SARS are both characterized by nosocomial outbreaks, MERS does not seem to spread as easily as SARS, the largest nosocomial outbreak for MERS up to now being 1 patient causing 23 secondary cases in linked hospitals in Al Hasa, Saudi Arabia38. So based upon the incidence, R0 and scale of reported nosocomial outbreaks, MERS-CoV can be considered less of a risk compared to SARS-CoV. The mortality rate of SARS-CoV (10%) was considerably lower compared to MERS-CoV today (40%), increasing the risks associated with MERS-CoV compared to SARS-CoV. This might be partly due to MERS-CoV infection often happening in people with underlying diseases and/or improper diagnostic tools and measures to diagnose mild or asymptomatic MERS cases. Nevertheless, proper measures should be taken for handling and containing MERS-CoV infection, since the origin and mode of transmission of the virus are still not known, in contrast to SARS-CoV, which was contained within a year due to the known origin, intermediate hosts and mode of transmission. Comparing the epidemiological data available for MERS and SARS, SARS-CoV has an eruptive character though relatively easily containable, whilst MERS-CoV has a slowly progressing but sustaining character, not necessarily being less of a threat. Origin of MERS- and SARS-CoV MERS- and SARS-CoV both belong to the genus Betacoronavirus and therefore share a relative close phylogenetic relationship based upon genetic analyses of the genome (figure 1). Within the genus Betacoronavirus they are classified into different lineages, lineage C and B respectively, each containing a unique set of accessory genes. A search for the origin of SARS-and MERS-CoV has been adopted in the past and present in order to contain spread of infection of both viruses and prevent future outbreaks. For the outbreak of SARS late 2002, Himalayan palm civets (Paguma larvata), raccoon dogs (Nyctereutes procyonoides) and Chinese ferret badgers (Melogale moschata), present at live animal markets in Southern China, were pointed out as the immediate source of human infection10-12. SARS-CoV like viruses were collected from these animals and genome sequence analyses confirmed up to 99.8% homology with the human SARS-CoV, with most differences in the Sgene sequences relating to receptor binding10. However, the true reservoir for SARS-CoV was discovered two years later. Horseshoe bats (Rhinolophus), also present at the live animal markets of Southern China, contained SARS-CoV like viruses with a 92% homology with human SARS-CoV, with 6 A risk assessment of MERS-CoV compared to SARS-CoV most differences in the S1 domain of the S gene, again relating to receptor binding6. Recently, two novel SARS-CoV like viruses were isolated from Chinese horseshoe bats with 95% homology to human SARS-CoV, in addition to the isolation of live SARS-CoV like virus from the same species with the ability to use the ACE2 receptor from humans, civet cats and horseshoe bats46. The latter finding may implicate that intermediate hosts may not have been necessary for cross-over of SARS-CoV to humans. In search for the origin of MERS-CoV, bats have been suggested as a primary source7,8,47,48. MERS-CoV like viruses were found in many bat families by means of extended RNA dependent RNA polymerase (RdRp) fragment analyses of viruses, isolated from a variety of specimens collected from different bats species from Saudi-Arabia, Africa, Asia, Europe and the Americas. Recently, virus isolated from one 1 out of 29 captured Taphozous perforates bats (family Emballonuridae) in Saudi-Arabia showed 100% nucleotide identity with a MERS-CoV isolated from a MERS patient living in the same region9. Besides the bat family Emballonuridae, other bat families identified with MERS-CoV like viruses outside Saudi-Arabia include Nycteridae, Vespertilionidae and Molosidae,7,47,48. Within these bat families, viruses belonging to Betacoronavirus line age C were found in Nycteris bats from Ghana, in Pipistrellus bats from Europe, in a Nyctinomops laticaudatus bat in Mexico and in a Neoromicia cf. zuluensis bat in South-Africa7,47,48. These MERS-CoV like viruses, together with HKU4 and HKU5 originating from Pipistrellus and Tylonycteris bats in China, are closely related to MERS-CoV (figure 5)7,49,50. Fig 5 Origin of MERS-CoV: close phylogenetic relationship of MERS-COV (EMC/2012) to bat coronaviruses from Saudi-Arabia, Africa, Europe and China. MERS-CoV shows phylogenetic relationship with bat coronaviruses from Saudi-Arabia (in red), Europe (in yellow), China (in green) and Africa (in blue).The isolate from the Taphozous perforates bat in Saudi-Arabia (upper red box) showed 100% nucleotide similarity with MERS-CoV9. 7 A risk assessment of MERS-CoV compared to SARS-CoV In support of a bat origin of MERS-CoV, Cui et al., 2013, found evidence of long term co-evolution between bats and MERS-CoV by studying the molecular evolution of DDP4 across a variety of mammalian species8. However, the seemingly great number of sporadic cases and introductions of different MERS-CoV genotypes22,36, without a history of prior contact with bats, gives rise to the likelihood of an existing intermediate host such as camels, goats and other cattle present in SaudiArabia and surroundings. The spreading of bat fomites or urine on fruits such as dates, as was the primary mode of transmission for Nipah virus from bats to the people of Bangladesh51, also needs to be taken into consideration. The last option would give a possible explanation of the genetic diversity of MERS-CoV isolated from clusters in geographically confined areas. The fact that the immediate source of infection was known for SARS-CoV back in 2002/2003, helped to contain the spread of infection. Live animal trade at the markets in Guangdong, Southern China was banned, which ended the first outbreak of SARS in July 200311. A lift of the ban in September 2003 led to an additional four human SARS cases within two and a half months, imposing a permanent ban on live animal trade in Guangdong11. Since then, there has been no report of human SARS cases. Because no immediate source of infection has been identified for MERS-CoV, despite the suggestion of camels13-15, there remains a continuing risk of infection with MERS-CoV with new outbreaks to be expected. The large coronavirus genomes are prone to mutations due to the unreliable RNA dependent RNA polymerase and because of likely recombination events during replication2,12. This way, MERS-CoV might evolve enhanced human-to-human transmission when it continues to cross over from animal to human, especially when it has the time to evolve. Transmission, tropism and pathology In order to make a proper risk assessment of viral infection, the route of transmission, receptor usage and cellular tropism needs to be known. In the case of SARS, SARS-CoV is spread primarily via droplets and aerosols, infecting the respiratory epithelium11,18. Also fomites, infecting the gastrointestinal tract, may have contributed to SARS-CoV infection. SARS-CoV enters permissive host cells by an interaction of its S protein with the ACE2 receptor. This receptor is a metalloprotease present on cells of the respiratory tract, small intestines and kidney of humans, civet cats and other intermediate hosts, and also of bats11,12,17,18. However, the ACE2 receptor of horseshoe bats did not give entry to SARS-CoV, suggesting that bats use a different receptor for SARS-CoV entry and adaptations were necessary to infect a host with a different receptor12. The recent discovery and isolation of a SARS-CoV like virus from a horseshoe bat with the ability to use the ACE2 receptor of bats, civet cats and humans, suggests that no such adaptations were necessary after all46. The ability to infect and replicate in a specific host which harbors ACE2 is primarily dependent on the receptor binding domain (RBD) of the S protein. It was determined that primarily two amino acid changes in the RBD of the S protein; residues 479 and 487, changed host tropism from civet cats to humans 12. Human to human transmission for SARS-CoV was proven very efficient with the N479 and T487 combination in the RBD of the S protein52,53. MERS-CoV appears to use a different receptor to enter host cells; the DPP4 receptor present on primarily non-ciliated bronchial epithelium and kidney cells of bats, possible intermediate hosts (camels) and humans16. In contradiction to SARS-CoV, MERS-CoV is able to infect bat cells54 , extending the host tropism and cross-over possibilities of MERS-CoV, if bats were the original hosts of MERS-CoV. Thus far, MERS-CoV has not yet developed efficient human to human transmission, limiting the spread of the virus as opposed to the SARS-CoV spread back in 2003. The route of transmission is thought to be similar to SARS-CoV, which is primarily through respiratory droplets3, even though the oral route and transmission via urine cannot be excluded. Ex-vivo cultures of bronchial and lung tissue, as well as infection of primary and continuous cell lines of the human airways, demonstrated the broad and similar tropism of MERS-CoV compared to SARSCoV55,56. MERS-CoV even extends the tropism from differentiated human airway epithelium and primarily alveolar type II pneumocytes, to undifferentiated lung epithelium and alveolar type I 8 A risk assessment of MERS-CoV compared to SARS-CoV pneumocytes. MERS-CoV appeared to infect the non-ciliated bronchial epithelial cells, in line with the presence of the receptor on these cells16, 55. The ability of both SARS- and MERS-CoV to infect human alveolar epithelium correlates to the severe human lung pathology caused by both viral infections. MERS-CoV was also shown to infect the endothelial cells of interstitial blood vessels of infected ex vivo lung tissue cultures, which may cause systemic spreading of the virus55. For SARS-CoV systemic spread was already confirmed11, adding to the severity of the disease. Furthermore, MERS-CoV has the ability to infect kidney cells like SARS-CoV, which may account for the acute renal failure present in MERS patients, and also, even though less commonly, in SARS patients3,57. Comparative infection of primary human kidney cells showed cytopathogenic effect, however, only after MERS-CoV infection58. MERS-CoV RNA, but not the virus, was found in the urine of a human patient admitted in a clinic in Germany57. Therefore, transmission of MERS-CoV via urine, which was not established for SARS-CoV, should be taken in to consideration and further investigated. So, based upon the known cellular and tissue tropism as well as the mode of transmission, MERS-and SARS-CoV appear to behave similarly. They are both capable of infecting human bronchial epithelium, causing severe lung pathology and are able to infect human kidney cells, which may cause acute renal failure. Furthermore, systemic spread was shown for SARS-CoV and also seems to be possible for MERS-CoV. However, MERS-CoV seems to have an extended cellular and host tropism compared to SARS-CoV and a far more limited human to human transmission. The latter finding makes MERS-CoV infection less of a threat, even though the unidentified primary and intermediate host(s) enhance the possibility of cross-over to humans with evolvement of enhanced human to human transmission. The cross-over from animals to humans seems to be easier for MERS-CoV, because in contrast to SARS-CoV, which needed amino acid changes is the RBD of the S protein to develop human to human transmission52,53, these changes do not seem to be necessary for MERSCoV. Immune response, immune evasion and therapy To finalize a comparison of risk assessment between SARS-CoV- and MERS-CoV infection, host immune responses, host immune evasion strategies and therapeutic possibilities are evaluated for both viral infections. An appropriate immune response to mammalian viruses starts with an innate immune response, resulting in the production of cytokines, chemokines and interferons. In turn, interferon α and β (IFNα and β) activate, via autocrine and paracrine pathways, the transcription of interferon stimulating genes (ISG), resulting in an anti-viral state. SARS-CoV infection is characterized by a disturbed innate immune response with an increased production of cytokines and chemokines but a delayed and decreased interferon (IFN) and ISG response55,59. Specifically, interleukines IL-1β, IL-6 and IL-12 are up regulated excessively, as well as chemokines MCP-1 and IP-10, which attract monocytes and neutrophils respectively11,59. This aberrant up regulation of interleukines and chemokines can result in ARDS, associated with SARS in one fourth of the cases33. The delayed and decreased IFN response in SARS-CoV infection is a result of IFN antagonism by several SARS-CoV proteins as reviewed by Totura et al., 2012 and Perlman & Netland, 2009. These proteins include nsp1, nsp3, nsp7, nsp15, the N and M protein and accessory protein ORF6 and ORF3b18,59. Under normal circumstances, foreign (viral) RNA is detected by Rig I Like Receptors (RLR), which signal to kinases to phosphorylate the transcription factor IRF3, which is then transported into the nucleus to transcribe type I interferons (IFNα and β). IFNα and β bind to interferon receptors, which via JAK/STAT signalling phosphorylate transcription factors STAT1/2, which are subsequently transported into the nucleus to transcribe ISG59. The SARS-CoV proteins antagonize the IFN response in the majority of the cases by blocking signalling of transcription factors IRF3 or STAT1. The antagonized IFN response makes SARS-CoV infection not that responsive to IFNα and β treatment as shown by SARS-CoV infections performed on ex-vivo lung tissue cultures55 and in vitro cell lines56. 9 A risk assessment of MERS-CoV compared to SARS-CoV Instead, other therapeutic possibilities have been explored for SARS-CoV infection. Cyclosporin A (CsA) was discovered as a broad range coronavirus inhibitor60,61 (see also review by Hilgerfeld & Peiris, 2013), which blocks the interaction between a host isomerase and the N protein. Also, active and passive immunization strategies were explored. Active immunization with RNA and DNA vaccines based on the S-protein were found to give protection in animal models and passive immunization with monoclonal antibodies also seemed to give partial protection in animal models11,62. However, due to the containment of the SARS epidemic at the end of 2003, pharmaceutical companies were no longer interested in developing vaccines or drugs against SARSCoV. This short-sightedness or perhaps better said, flaw in our drug-development system, resulted in no available therapy for SARS-CoV even today. The new outbreak of MERS-CoV has boosted screens for new SARS-CoV and MERS-CoV inhibitors63,64. Adedeji et al., 2013 found new inhibitors of SARSCoV replication, acting by 3 distinct mechanisms: blocking the S-protein and ACE2 receptor interaction, blocking cathepsin L and blocking fusion of the viral membrane with the host cellular membrane. Whether some of these inhibitors are cross-reactive against MERS-CoV, remains to be investigated. The innate immune response after MERS-CoV infection was found to be partially disturbed in a similar way as with SARS-CoV infection, showing a down regulation of the IFN response55,56. Zielecki et al., 2013, found IRF3 to be retained in the cytoplasm upon MERS-CoV infection, similarly as upon SARS-CoV infection, resulting in a down regulation of IFN and ISG response. However, in MERS-CoV infected cells it was found, in contrast to SARS-CoV infection, that there is still translocation of phosphorylated STAT1 (p-STAT1)64. This finding coincides with the fact that MERS-CoV does not contain the equivalent accessory protein of SARS-CoV, ORF6, which blocks the nuclear translocation of p-STAT1 and thereby the induction of ISG64. Despite the partial blocking of the IFN response upon MERS-CoV infection, like upon SARS-CoV infection, it was found that MERS-CoV was better responsive (50-100 fold) to Polyethylene glycol-IFN and IFN-α or IFN-β treatment in vitro and in ex vivo lung tissue cultures compared to SARS-CoV55,56,64. Also the ‘’pan-coronavirus’’ inhibitor CsA was tested and found effective against MERS-CoV infection in vitro, even though no complete block of replication was obtained64. The responsiveness of MERS-CoV to IFNs and CsA generates hope for treatment possibilities for MERS-CoV in the future, if this virus is not contained by other measures. Based upon therapeutic responsiveness tested so far, MERS-CoV infection seems less of a threat compared to SARS-CoV infection, even though it is probably too early to make such a vast statement. Discussion Two coronaviruses have caused severe respiratory symptoms of the lower respiratory tract in humans in the last decade; SARS-CoV causing a pandemic back in 2003 and MERS-CoV causing cluster outbreaks at the moment in the Middle East. When MERS-CoV emerged in the summer of 2012 in Saudi-Arabia with similar clinical symptoms, pathology and apparent human to human transmission22, fear of a new pandemic arose. Here, a risk assessment of MERS-CoV compared to SARS-CoV was made, to get a clearer view of the threats associated with the new outbreak of MERSCoV and how to handle it. Based upon available epidemiological data, knowledge about the origin and spread of the viruses, and immune evasion strategies and therapy possibilities, it can be concluded at this moment in time that MERS-CoV poses less of a threat compared to prepandemic SARS-CoV back in 2002. MERS-CoV does not seem to efficiently spread between humans and reported clusters in the Middle East seem to consist of several viral introductions, indicating small transmission trees35,36. The mortality rate of MERS-CoV, reaching approximately 40%22, is very striking, but this might be an overestimation due to unreported mild or asymptomatic cases of MERS-CoV. In contrast, SARS-CoV was able to spread very rapidly between humans with a case fatality rate of 10%21. MERS-CoV also seems to be better responsive to interferons55,56,64 than SARSCoV due to the presence of fewer immune evasion strategies, which might generate better treatment possibilities for MERS in the future. 10 A risk assessment of MERS-CoV compared to SARS-CoV What needs to be taken into consideration, is that the knowledge available for MERS-CoV compared to SARS-CoV is far less. Not only have researchers been able to gain information about SARS-CoV in the past ten years compared to just one year for MERS-CoV, but the communication from the Middle East to the West about MERS-CoV has thus far been limited. The communication from China to the West about SARS-CoV back in 2003 was difficult in the beginning as well, but soon China became very open and cooperative with the West in the battle against SARS. The lack of communication in the case of MERS, together with the often poor surveillance and diagnostics of MERS in the Middle East32, make a good comparison of risks associated with SARS-and MERS-CoV difficult. At this moment in time, the origin and mode of transmission of MERS-CoV, still need to be established. For this to happen, accurate case reports, a history of case-contacts and proper diagnostics are crucial, as well as a screening of the general population for the presence of circulating MERS-CoV, to identify asymptomatic cases. Several animal reservoirs are being explored at the moment in the search for the origin of MERS-CoV, including bats7,9,47,48, as was the primary animal reservoir of SARS-CoV and camelsas a possible intermediate host reservoir13-15. The more information will become available for MERS-CoV, the better a risk assessment of MERS-CoV can be made. The challenge will lie in gathering and using the information appropriately, before new outbreaks with enhanced human to human transmission of MERS-CoV will occur. Recently, two types of influenza, influenza H5N1 and H7N9, have also crossed the species barrier in the new millennium from animals (poultry) to humans and have gained much public attention as potential new threats of global viral epidemics. Until now, neither of the two viruses has, however, evolved substantial human to human transmission. Judged on our risk assessment of MERS-CoV compared to SARS-CoV, MERS-CoV should at least gain as much public attention as influenza, not to raise fear, but to stimulate collaborative research in trying to prevent such zoonotic events. Acknowledgements The author would like to thank Berend Jan Bosch for the useful suggestions while writing this thesis, making it a smooth and relative easy writing experience. References 1. Virus Taxonomy (eds King, A. M. Q., Lefkowitz, E., Adams, M. J. & Carstens, E. B.) 806-828 (Elsevier, San Diego, 2012). 2. Woo, P. C., Lau, S. K., Huang, Y. & Yuen, K. Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. (Maywood) 234, 1117-1127 (2009). 3. Guery, B. et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet (2013). 4. Zaki, A. M., van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. & Fouchier, R. A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814-1820 (2012). 5. de Groot, R. J. et al. Middle East Respiratory Syndrome Coronavirus (MERS-CoV); Announcement of the Coronavirus Study Group. J. Virol. (2013). 6. Li, W. et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 310, 676-679 (2005). 7. Annan, A. et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg. Infect. Dis. 19, 456-459 (2013). 11 A risk assessment of MERS-CoV compared to SARS-CoV 8. Cui, J., Eden, J. S., Holmes, E. C. & Wang, L. F. Adaptive evolution of bat dipeptidyl peptidase 4 (dpp4): implications for the origin and emergence of Middle East respiratory syndrome coronavirus. Virol. J. 10, 304-422X-10-304 (2013). 9. Memish, Z. A. et al. Middle East respiratory syndrome coronavirus in bats, saudi arabia. Emerg. Infect. Dis. 19, 10.3201/eid1911.131172 (2013). 10. Guan, Y. et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276-278 (2003). 11. Peiris, J. S., Guan, Y. & Yuen, K. Y. Severe acute respiratory syndrome. Nat. Med. 10, S88-97 (2004). 12. Li, W. et al. Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions. J. Virol. 80, 4211-4219 (2006). 13. Haagmans, B. L. et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. (2013). 14. Reusken, C. B. et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 13, 859-866 (2013). 15. Perera, R. A. et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 18, pii=20574 (2013). 16. Raj, V. S. et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495, 251-254 (2013). 17. Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450-454 (2003). 18. Perlman, S. & Netland, J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 7, 439-450 (2009). 19. Parashar, U. D. & Anderson, L. J. Severe acute respiratory syndrome: review and lessons of the 2003 outbreak. Int. J. Epidemiol. 33, 628-634 (2004). 20. Kindler, E. et al. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. MBio 4, e00611-12 (2013). 21. WHO. Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS). http://www.who.int/csr/sars/country/en/. Accessed December 10, 2013. 22.WHO. MERS-CoV summary and literature update-as of 22 November, 2013. http://www.who.int/csr/disease/coronavirus_infections/archive_updates/en/. Accessed December 13, 2013. 23. Bosch, B. J., van der Zee, R., de Haan, C. A. & Rottier, P. J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 77, 8801-8811 (2003). 24. Matsuyama, S., Ujike, M., Morikawa, S., Tashiro, M. & Taguchi, F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. U. S. A. 102, 12543-12547 (2005). 25. Simmons, G. et al. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 102, 11876-11881 (2005). 26. Bosch, B. J., Bartelink, W. & Rottier, P. J. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 82, 8887-8890 (2008). 27. Bertram, S. et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 85, 13363-13372 (2011). 12 A risk assessment of MERS-CoV compared to SARS-CoV 28. Huang, I. C. et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 281, 3198-3203 (2006). 29. Shirato, K., Kawase, M. & Matsuyama, S. Middle East Respiratory Syndrome Coronavirus Infection Mediated by the Transmembrane Serine Protease TMPRSS2. J. Virol. 87, 12552-12561 (2013). 30. Gierer, S. et al. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J. Virol. 87, 5502-5511 (2013). 31. de Groot, R. J. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj. J. 23, 59-72 (2006). 32. de Sousa, R., Reusken, C. & Koopmans, M. MERS coronavirus: Data gaps for laboratory preparedness. J. Clin. Virol. (2013). 33. Lew, T. W. et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 290, 374-380 (2003). 34. WHO. MERS-CoV summary and literature update- as of 20 June 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130620/en/. Accessed December 13, 2013. 35. Breban, R., Riou, J. & Fontanet, A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet (2013). 36. Cotten, M. et al. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet (2013). 37. Penttinen, P. M. et al. Taking stock of the first 133 MERS coronavirus cases globally--Is the epidemic changing? Euro Surveill. 18, 20596 (2013). 38. Assiri, A. et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 369, 407-416 (2013). 39. Cauchemez, S. et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect. Dis. 14, 50-56 (2014). 40. Lipsitch, M. et al. Transmission dynamics and control of severe acute respiratory syndrome. Science 300, 1966-1970 (2003). 41. Cooper, B. S. et al. Transmission of SARS in three Chinese hospitals. Trop. Med. Int. Health 14 Suppl 1, 71-78 (2009). 42. Lee, N. et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348, 1986-1994 (2003). 43. Wu, W. et al. Comparison of clinical course of patients with severe acute respiratory syndrome among the multiple generations of nosocomial transmission. Chin. Med. J. (Engl) 117, 14-18 (2004). 44. Lipsitch, M. et al. Transmission dynamics and control of severe acute respiratory syndrome. Science 300, 1966-1970 (2003). 45. Cheng, V. C., Chan, J. F., To, K. K. & Yuen, K. Y. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 100, 407-419 (2013). 46. Ge, X. Y. et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503, 535-538 (2013). 47. Ithete, N. L. et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg. Infect. Dis. 19, 1697-1699 (2013). 48. Anthony, S. J. et al. Coronaviruses in bats from Mexico. J. Gen. Virol. 94, 1028-1038 (2013). 49. van Boheemen, S. et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 3, 10.1128/mBio.00473-12 (2012). 13 A risk assessment of MERS-CoV compared to SARS-CoV 50. Chan, R. W. & Poon, L. L. The emergence of human coronavirus EMC: how scared should we be? MBio 4, e00191-13 (2013). 51. Luby, S. P., Gurley, E. S. & Hossain, M. J. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 49, 1743-1748 (2009). 52. Cheng, V. C., Lau, S. K., Woo, P. C. & Yuen, K. Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 20, 660-694 (2007). 53. Qu, X. X. et al. Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J. Biol. Chem. 280, 29588-29595 (2005). 54. Muller, M. A. et al. Human coronavirus EMC does not require the SARS-coronavirus receptor and maintains broad replicative capability in mammalian cell lines. MBio 3, 10.1128/mBio.00515-12 (2012). 55. Chan, R. W. et al. Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. J. Virol. 87, 6604-6614 (2013). 56. Zielecki, F. et al. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J. Virol. 87, 5300-5304 (2013). 57. Drosten, C. et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect. Dis. 13, 745-751 (2013). 58. Eckerle, I., Muller, M. A., Kallies, S., Gotthardt, D. N. & Drosten, C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol. J. 10, 359-422X-10-359 (2013). 59. Totura, A. L. & Baric, R. S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2, 264-275 (2012). 60. Pfefferle, S. et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 7, e1002331 (2011). 61. de Wilde, A. H. et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 92, 2542-2548 (2011). 62. Gillim-Ross, L. & Subbarao, K. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 19, 614-636 (2006). 63. Adedeji, A. O. et al. Novel Inhibitors of SARS-CoV Entry acting by Three Distinct Mechanisms. J. Virol. (2013). 64. de Wilde, A. H. et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J. Gen. Virol. (2013). 14