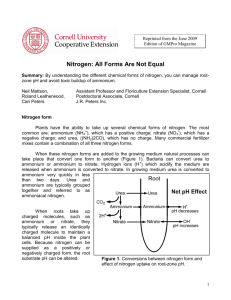
conversion of N 2 into ammonia, and thence into proteins, is
advertisement

Nitrogen Cycle terms to know Nitrogen fixation conversion of N2 into ammonia, and thence into proteins, is achieved by microorganisms in the process called nitrogen fixation (or dinitrogen fixation). Denitrification The biological reduction of nitrate to nitrogen gas by denitrifying bacteria in soil. Occurs in oxygen poor environments Nitrification Oxidation of ammonium (NH4+) ions into nitrite (NO2-) ion and then nitrate (NO3-) ions by microorganisms in soil and water. Nitrate ions are absorbed by the plants as essential nutrients and, with the help of oxygen, converted (synthesized) into plant protein (amino acids). See also nitration. Assimilation when a plant incorporates nitrogen into its tissues, making proteins and nucleic acids Ammonification the conversion of organic nitrogen to ammonium (NH4+) by the action of decomposers (bacteria). Occurs in oxygen poor environments Ammonification is an important stage in the nitrogen cycle, a natural cycle which makes the Earth's supply of this essential element available to living organisms. It is carried out by a variety of microorganisms found in soil and water, which break down proteins and amino acids in dead plant and animal matter, and feces, releasing ammonia, which is usually retained in soil or water in the form of the ammonium ion. Other groups of microorganisms then convert this into nitrate, which can be absorbed by plants, maintaining the cycle. Ammonification is therefore essential to all plant and animal life on the planet. In agriculture and horticulture, the addition of compost and manure to soil provides an extra source of nitrogen for ammonification Ammonification is the process — carried out by a variety of microorganisms — that breaks down proteins, amino acids, and other nitrogen-containing compounds in dead and waste organic matter to form ammonia. Proteins are first split up into amino acids, which are compounds containing an amine (NH2) group by bacteria using enzymes known as proteases. The amino acids, and other compounds with amine groups, such as nucleic acids and urea, are then decomposed by microorganisms known as ammonifying bacteria, releasing ammonia (NH3). This dissolves in water, and usually forms ammonium (NH4+) ions, by combining with hydrogen (H+) ions, which are abundant in most soils. This ammonium is oxidized to nitrites and nitrates by nitrifying bacteria, in the same way as nitrogen that has been “fixed” from the atmosphere.