Current Issues in Engineering related to Engineering Ethics
advertisement

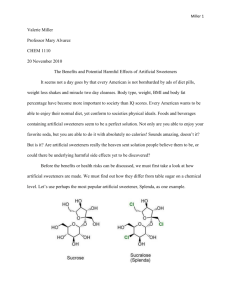
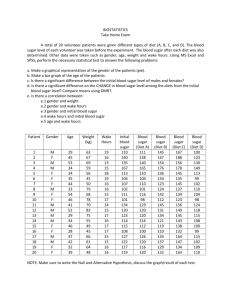
ENGR 0011 Bursic 2:00 R03 ASPARTAME: THE SWEET SAFE AND LOW CALORIE ALTERNATIVE TO SUGAR Matthew Sykes (mss110@pitt.edu) ASPARTAME: SWEET, SAFE SATISFACTION WITHOUT THE CALORIES At some point in their life, everyone feels an innate craving for something sweet. Some call this having a “sweet tooth.” However, this instinct can be attributed to the necessary role sugar plays in the human body. On average, adult Americans eat 22 teaspoons of sugar per day, while teens eat 34 teaspoons per day [5]. This is a very large amount considering the recommended amount of sugar for an adult man of normal weight is 9 teaspoons and 6 teaspoons for a woman. Cutting down on sugar intake can benefit the body; however, completely removing sugar from one’s diet can cause an individual to become confused, forgetful and even lapse into a coma [5]. Finding the proper balance can be difficult, causing many people to turn to sugar substitutes, or artificial sweeteners, to satisfy a sweet tooth. Because these products are compounds much sweeter than sucrose, common table sugar, much less sweetener is required, thus energy contribution is often negligible [2]. However, some people are under the belief that artificial sweeteners are detrimental towards the human body. As a child my family would constantly tell me that diet sodas, and any other products with sugar alternatives, could cause harm and even cancer. These products were never allowed in my home. For a while I accepted this twisted belief; however, as I got older I began to question how a product so widely marketed could cause so much harm. I knew there must be some set of moral obligations engineers hold in order to preserve the health and wellbeing of society. If artificial sweeteners were detrimental towards one’s health, engineers around the world would have been obligated to remove it from the market in order to truthfully “hold paramount the safety, health, and welfare of the public” [9]. Engineers are expected to exhibit the highest standards of honesty and integrity because every action they make, every product they develop, has “a direct and vital impact on the quality of life for all people” [9]. On an almost daily basis, my mother would tell me how unsafe any product containing artificial sweeteners were; however, I am certain that chemical engineers worldwide have ensured that artificial sweeteners provide a safe, healthy and efficient sugar alternative. Without the in-depth research and comprehensive reading on engineering ethics and its daily application, I would not be as well informed as I am now. I might have even second guessed myself about the safety of sugar alternatives, therefore siding with the uninformed opinions University of Pittsburgh, Swanson School of Engineering 1 10/28/12 of my mother. These uniformed individuals as well as many others might argue against incorporating ethics education into a freshman engineering curriculum. However, they most likely believe ethics education is simply teaching common morality, a set of principles defining “right” from “wrong.” Why would any educational institution waste their time teaching their students the difference between right and wrong when this concept is instilled in students at a young age? I strongly believe incorporating engineering ethics into a freshman curriculum is not a waste of time. It is an invaluable asset that should be a part of every engineering school’s curriculum [11]. The ultimate aim of efforts to teach engineering ethics is not to produce moral engineers, but rather to instill clarity of insight and strong decisionmaking skills. “Engineering ethics is not teaching right or wrong, or good or bad, but rather methods of making personal decisions about these qualities” [11]. Common morality is “taught” in a sense that it is instilled in primarily young individuals, and constitutes expected behavior by certain groups of people or individuals. On the other hand, adherence to the codes of professional ethics must be willfully adopted by the professionals as they enter their specific field. Without the incorporation of engineering ethics education into a freshman curriculum, such as a project like this, I would not be closer to achieving cognitive goals in my knowledge and reasoning. Through my research for this assignment and future projects I will be better able to address society’s problems while “conducting [myself] honorably, responsibly, ethically, and lawfully” [9]. Without educating students on ethics, the chemical engineers responsible for the production of artificial sweeteners might have taken a shortcut, or left information out of a public statement which could have caused widespread suffering. However, these engineers stayed loyal to their code, ensuring the safety of sugar alternatives. I am confident that incorporating artificial sweeteners into commercial products does not pose a significant enough health risk to remove them from the market. However, if artificial sweeteners did pose a health risk, the world would have been well informed by the 21st century. The first artificial sweetener, saccharin, was discovered in 1879 when Constantin Fahlber, while working on coal-tar derivatives, noticed a substance on his hands and arms that tasted sweet. The world of artificial sweeteners began when Fahlber licked this substance off his body [1]. Saccharin began to grow in popularity and eventually was used to sweeten foods during sugar rationings in World War Matthew Sykes I and II. There were no adverse health effects of saccharin, just a slight metallic aftertaste [2]. As a result, scientists developed cyclamate, an artificial sweetener that had a more natural taste than saccharin. By 1968, food and beverage companies jumped at the opportunity to sweeten their products with something that is more natural tasting. At this point, Americans consumed more than 17 million pounds of the calorie-free substance a year in snack foods, canned fruit and soft drinks [1]. However, in the late 1960’s studies began linking cyclamate to cancer. One study showed that chicken embryos injected with the chemical developed extreme deformities. Another study linked the sweetener to malignant bladder tumors in rats [1]. Knowing this, chemical engineers adhered to the American Institute of Chemical Engineers by “formally advising their employers [about a consequence of their duties that would] adversely affect the present and future health and safety of the public” [10]. It is a result of the implementation of engineering ethics education that these engineers were able to think ethically and address this problem so as to benefit the population. In this case, engineers were not the only ones to act ethically, the government also took action to correct the problem. Because a 1958 congressional amendment required the Food and Drug Administration (FDA) to ban any food additive shown to cause cancer in humans or animals, on October 18th 1969, the government ordered the removal of cyclamate from all food products [1]. The government has been working together with scientists since artificial sweeteners’ introduction in the 18th century to ensure that there are no serious adverse effects. Problems, such as cyclamate, were solved early in the development of sugar alternatives. Artificial sweeteners’ development continued into the 1980’s when the synthetic compound aspartame was approved for use, later becoming the leading additive in diet colas [2]. amino acids L-aspartic acid and L-phenylalanine, see figure 1 [4], [7]. This chemical was the leading cause of fear among my family. My mother told me that the aspartame would cause serious health problems and could even lead to cancer. As a result, I have only had diet cola a few times in my life and have now acquired a strong distaste towards it. However, if this chemical is so dangerous, why is it around, I asked my mother. She told me not to ask questions because she knows what is best for me. There have been many industry conspiracies surrounding aspartame that have been circulated around the internet and the news, few having reached my mother’s ears. Conflicts of interest in the studies performed on aspartame and the study’s methodology is an ongoing controversy. Dr. Robert Walton surveyed the studies of aspartame and found that of the 166 studies to have relevance for questions of human safety, 74 had Nutrasweet industry, those who produce aspartame, funding and 92 were independently funded. One hundred percent of the research that was done by the Nutrasweet funded program confirmed aspartame’s safety, whereas ninety-two percent of the independently funded research discovered issues with ingesting aspartame [2]. It is research and publication materials such as these that violate the engineering code of ethics. Canon five of the code states that “engineers shall avoid deceptive acts. Engineers shall not falsify their qualifications or permit misrepresentation of their or their associate’s qualifications. They shall not misinterpret or exaggerate their responsibility in or for the subject matter of prior assignments” [9]. The engineers working on these studies were probably not acting in a way that would benefit the health and wellbeing of all people. These engineers/scientists most likely did not “issue public statements only in an objective and truthful matter” [9]. Instead, they acted dishonest and partial, providing information that would only appease the company that pays them. I explained to my mother that the men and women involved with such studies were most likely unethically influenced, yet, she still held fast to her beliefs. However, while my mother might still believe some of the information produced from such surveys, she also told me “everything in moderation.” As a future engineer, I can apply this rule to alternative sweeteners and confidently assert to my mother that aspartame is safe in certain dosages. The Food and Drug Administration (FDA) has set the acceptable daily intake (ADI) for aspartame at 50mg/kg of body weight [3]. To put this in perspective, this would be about 3,750mg of aspartame per day for a typical adult weighing 75kg, which is far greater than most adult’s daily intake. A typical can of diet cola contains about 180mg of aspartame, therefore a typical adult would have to drink about 21 cans of diet cola to come anywhere near the ADI limit for aspartame [3]. It is partially because of the dedicated chemical engineers that there is an ADI for aspartame. These engineers “acted for each employer as faithful agents…disclosing all known or potential conflicts of interest that could influence or appear FIGURE 1, ASPARTAME CHEMICAL STRUCTURE The chemical structure of aspartame, the leading alternative “general purpose sweetener,” which can be found in more than six thousand food products [7]. Aspartame, as well as being used in diet cola, is also known as Nutrasweet, Equal, and Sugar Twin [3]. Aspartame is a methyl ester of the dipeptide of the natural 2 Matthew Sykes to influence their judgment or the quality of their services” [9]. By sticking to their code of ethics, engineers were able to partner with the FDA and other companies worldwide in order to improve the general public’s quality of life. This collaboration led to aspartame being approved for use in over 100 countries. The European Food Safety Authority (EFSA) asserted the safety of sweeteners, such as aspartame, throughout the European Union. According to a 2009 report from its Panel on Food Additives and Nutrient Sources Added to Food, they claimed that: “Overall, the Panel concluded, on the basis of all the evidence currently available…that there is no indication of any genotoxic or carcinogenic potential of aspartame and that there is no reason to revise the previously established ADI for aspartame” [3]. Although aspartame is safe for the majority of the population, scientists from the FDA and EFSA have concluded that aspartame is not perfect. Phenylketonuria is a rare genetic disorder, present at birth, in which the body cannot break down phenylalanine, an amino acid found in many foods. Levels of phenylalanine can build up in the blood, preventing other important chemicals from getting to the brain. Unless phenylalanine intake is kept to a certain level, children with phenylketonuria can suffer from abnormal brain development. Because phenylalanine is a component of aspartame, it is very important that people, especially children, keep their intake of aspartame to a minimum [3], [4]. However, because the occurrence of phenylketonuria is so small, about one in every fifteen-thousand children in the United States per year, removing aspartame and other artificial sweeteners from food products would not be worth the trouble, considering it can be found in over six-thousand products [3], [1]. Thus, people with Phenylketonuria simply follow a phenylalanine-restricted diet. However, phenylalanine is only one of the three main components of aspartame that have been claimed to cause adverse health effects. professor of neurosurgery at the Medical University of Mississippi, recently published a book discussing how the ingestion of excessive aspartic acid from aspartame can cause damage [5]. Aspartate acts as neurotransmitters by aiding in the transmission of information from neuron to neuron. However, too much aspartate in the brain kills certain neurons by allowing the influx of a high amount of calcium into the cells. Subsequently, this influx triggers excessive amounts of free radicals, which in turn kill the cells. The neural cell damage that can be caused by excessive aspartate is why it is sometimes called an “excitotoxin” because it “excites” or stimulates the neural cells to death [5]. The excess aspartate slowly begins to destroy neurons with the large majority of neural cells in a particular area of the brain being killed before any clinical symptoms of a chronic illness are noticed. A few of the chronic illnesses that have been shown to be directly related to long-term exposure to excitatory amino acid damage include multiple sclerosis, memory loss, hormonal problems, Alzheimer’s disease and in an extreme case, brain lesions [5], [4]. However, all of these extreme side effects are caused by long-term high exposure to aspartame. Acceptable daily intakes have been set for a reason; human bodies cannot ingest unlimited quantities of these alternatives. Because sweeteners are not essential nutrients in our diet, they must be taken in strict moderation. This moderated intake level can replace a majority of the sugar intake that is necessary. This replacement satisfies the body’s instinctual drive for sugar, without the negative side effects associated with table sugar. For example, it has been proposed that consuming too much sugar suppresses the immune system for a period of time. The white blood cells that attack bacteria are less effective after an increase in sugar levels. Studies have shown that overweight mice create fewer antibodies after receiving vaccinations, thus illustrating a compromised immune system. Increased sugar levels are also an indirect cause of obesity diabetes [5], [6]. Studies have shown the correlation between refined sugar consumption and the onset of diabetes. For example, a 2010 meta-analysis of eleven studies involving 310,819 people and 15,043 cases of type-two diabetes found that sugarsweetened beverages may increase the risk of metabolic syndrome and type-two diabetes not only through obesity, but also by increasing the dietary glycemic load, which leads to an insulin resistance [5], [4]. However, the World Health Organization excluded sugar alternatives from this study, therefore only illustrating a correlation of free sugars, monosaccharaides and disaccharides, to adverse health effects [5]. But the risk of being diagnosed with one of these health effects can be lowered through the implementation of alternative sweeteners into one’s diet. With the correct balance of common table sugar, and artificial sweetener, one can live a healthy life without the fear of compromising one’s health. FIGURE 2, SUGAR ALTERNATIVE Sugar substitute packets are just one of the many modern products containing aspartame [8]. Like phenylalanine, aspartame is also made up of aspartic acid and methanol. Dr. Russell L. Blaylock, a 3 Matthew Sykes mother that aspartame, in moderation, is not harmful at all. There is nothing wrong with the right amount of diet cola. I might even find some at the next birthday party. THE CORRECT AMOUNT OF ASPARTAME PROVIDES A CLEAN ALTERNATIVE TO SUGAR REFERENCES In 2011 the world produced about 168 million tons of sugar, with the average person consuming about 24 kilograms of sugar each year [5]. This is an obscenely large number that, if the majority of people adhered to, could lead to many health conditions such as obesity, diabetes and a weak immune system. Chemical engineers have attempted to solve this problem with the introduction of artificial sweeteners into the market. By providing a substance that tastes like sugar, but does not contain the negative components, engineers have been able to satisfy the drive for sweetness without the health risks. Through a partnership with the Federal Government, they were able to set an acceptable daily intake for such alternatives. According to the FDA, “Regardless of whether the use of a substance is a food additive use or is GRAS (Generally Recognized as Safe), there must be evidence that the substance is safe under the conditions of its intended use. FDA has defined ‘safe’ as a reasonable certainty in the minds of competent scientists that the substance is not harmful under its intended conditions of use” [3]. Through strict government regulation and engineering prowess, we are able to provide a safe, sweet alternative to sugar. However, this engineering prowess must be kept within the ethical, moral boundaries outlined in the various codes of ethics for any positive, productive change to be implemented in the world. Morality, by itself, involves behavioral dispositions that may be encouraged, demanded or possibly behaviorally conditioned, but it cannot be “taught” in a traditional academic sense. What educators, and the creators of the codes of ethics, can hope for is to encourage “cognition and practice in a form of parity of reasoning” [11]. An old adage once said that if you give a man a fish you feed him for a day, but if you teach him to fish, you feed him for a lifetime. I believe this adage provides a great insight into the importance of incorporating engineering ethics into a freshman engineering curriculum. If an instructor tells his/her students what is or is not right in certain circumstances, than the instructors have only educated them about those specific circumstances. But, if engineering ethics education trains the engineers of tomorrow to analyze, think critically, and soundly evaluate, then these students have been educated for a lifetime [11]. This type of education will educate students so that they can naturally and effortlessly perform under a standard of professional and ethical behavior in their future endeavors. Engineering codes of ethics and the education of such ethics are largely responsible for producing the chemical engineers of today’s world. As a result of such education, they were able to undergo the proper steps in ensuring the safety of artificial sweeteners. I am now confident in telling my [1] Suddath, Claire. "Are Artificial Sweeteners Really That Bad for You?" Time. Time, 20 Oct. 2009. Web. 09 Oct. 2012. <http://www.time.com/time/health/article/0%2C8599%2C19 31116%2C00.html>. [2] Kovacs, Betty, MS, RD. "Artificial Sweeteners: Side Effects, Cancer Risk, Weight Gain and Pros and Cons of Sugar Substitutes by MedicineNet.com." MedicineNet. Ed. William C. Shiel, MD, FACP, FACR. N.p., 2011. Web. 09 Oct. 2012. <http://www.medicinenet.com/artificial_sweeteners/article.h tm>. [3] "Aspartame." Aspartame. National Cancer Institute, 17 Feb. 2011. Web. 09 Oct. 2012. <http://www.cancer.org/Cancer/CancerCauses/OtherCarcino gens/AtHome/aspartame>. [4] Ludwig D. Are Artificial Sweeteners a Good Alternative to Sugar? Harvard Health Letter [serial online]. December 2011;37(2):1. Available from: Academic Search Premier, Ipswich, MA. Accessed Octoer 9, 2012. [5] "Is Aspartame the Most Dangerous." Mercola Health. N.p., 2011. Web. 9 Oct. 2012. <http://articles.mercola.com/sites/articles/archive/2011/11/0 6/aspartame-most-dangerous-substance-added-tofood.aspx>. [6] Heikel, B., Krebs, E., Kohn, E., & Busch-Stockfisch, M. (2012). Optimizing Synergism of Binary Mixtures of Selected Alternative Sweeteners. Journal On Sensory Studies, 27(5), 295-303. Academic Search Premier, EBSCOhost Accessed October 9, 2012. [7] Aspartame. N.d. Photograph. Wikimedia Creative Commons. 2008. Web. 9 Oct. 2012. <http://en.wikipedia.org/w/index.php?title=File:Aspartame.s vg&page=1>. [8] "Aspartame Sugar Packet." Wikimedia Creative Commons. N.p., 2012. Web. 9 Oct. 2012. <http://en.wikipedia.org/wiki/File:Aspartame_sample.jpg>. [9] “Code of Ethics for Engineers." National Society of Professional Engineers. N.p., 2007. Web. 29 Oct. 2012. <http://www.nspe.org/resources/pdfs/Ethics/CodeofEthics/C ode-2007-July.pdf>. [10] “Code of Ethics." AIChE. American Institute of Chemical Engineers, n.d. Web. 29 Oct. 2012. <http://www.aiche.org/about/code-ethics>. [11] Abaté, Charles. "Should Engineering Ethics Be Taught?." Science & Engineering Ethics 17.3 (2011): 583596. Academic Search Premier. Web. 29 Oct. 2012. 4 Matthew Sykes ACKNOWLEDGMENTS I would personally like to thank Judy Brink for coming into class to help all of us in finding research. I would have struggled without her guidance. I would also like to thank Pat O’Donnel for reading/reviewing my essay. Being a junior engineering student, he has the wisdom to help better my paper. I would also like to thank Hans Mattingly for providing valuable insight and corrections on my previous paper. Finally, I would like to thank my peer advisor Katherine Colwell for continuing to help me through the process of completing a collegiate research paper. 5