7-206 InVitro Analytical Performance Characteristics and Clinical
advertisement

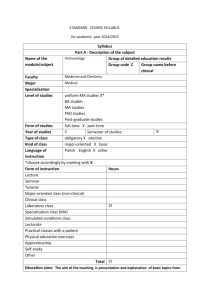
Analytical Performance Characteristics and Clinical Utility of Immunological Assays for Human 7-206 InVitro Immunoglobulin E (IgE) Antibodies I/LA20-A2 08/05/2013 CLSI and Defined Allergen Specificities; Approved Guideline - Second Edition Recognition List Number: 031 Publication Date: 08/05/2013 Part B: SUPPLEMENTARY INFORMATION Recognition Number 7-206: CLSI I/LA20-A2, Analytical Performance Characteristics and Clinical Utility of Immunological Assays for Human Immunoglobulin E (IgE) Antibodies and Defined Allergen Specificities; Approved Guideline - Second Edition. (InVitro Diagnostics) Date of Standard: 2009. Address of Standards Organization: Clinical Laboratory Standards Institute (CLSI) 950 West Valley Road Suite 2500 Wayne, PA 19087 CDRH Offices and Divisions Associated with Recognized Standards: (1) OFFICE OF IN VITRO DIAGNOSTICS AND RADIOLOGICAL HEALTH (OIR) (2) OFFICE OF DEVICE EVALUATION (ODE) DIVISION OF CARDIOVASCULAR DEVICES (DCD) Devices Affected: Immunological Assays for Human Immunoglobuline E (IGE) Antibodies of Defined Allergen Specificity Processes Affected: 510(k) Type of Standard: National, Vertical Extent of Recognition: Complete standard Related CFR Citations and Product Codes: Regulation Number Device Name Device Product Class Code §878.448021 Powder, Dusting, Surgical Class 3 KGP22 Regulation Device Product Number Device Name Class Code §866.551023 Ige, Antigen, Antiserum, Control Class 2 DGC24 §866.551025 Radioimmunoassay, Immunoglobulins (D, E) Class 2 JHR26 Regulation Device Product Number Device Name Class Code §866.575027 System, Test, Radioallergosorbent (Rast) Immunological Class 2 DHB28 Relevant Guidance: The Decision Summaries of recently cleared Allergy IVD devices in FDA database may be considered for use. Points to Consider for Collection of Data in Support of In-Vitro Device Submissions for 510(k) Clearance [Code of Federal Regulations] [Title 21, Volume 8] [Revised as of April 1, 2013] [CITE: 21CFR878.4480] TITLE 21--FOOD AND DRUGS CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES SUBCHAPTER H--MEDICAL DEVICES PART 878 -- GENERAL AND PLASTIC SURGERY DEVICES Subpart E--Surgical Devices Sec. 878.4480 Absorbable powder for lubricating a surgeon's glove. (a)Identification. Absorbable powder for lubricating a surgeon's glove is a powder made from corn starch that meets the specifications for absorbable powder in the United States Pharmacopeia (U.S.P.) and that is intended to be used to lubricate the surgeon's hand before putting on a surgeon's glove. The device is absorbable through biological degradation. (b)Classification. Class III. (c)Date PMA or notice of completion of a PDP is required. As of May 28, 1976, an approval under section 515 of the act is required before this device may be commercially distributed. See 878.3. [Code of Federal Regulations] [Title 21, Volume 8] [Revised as of April 1, 2013] [CITE: 21CFR866.5510] TITLE 21--FOOD AND DRUGS CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES SUBCHAPTER H--MEDICAL DEVICES PART 866 -- IMMUNOLOGY AND MICROBIOLOGY DEVICES Subpart F--Immunological Test Systems Sec. 866.5510 Immunoglobulins A, G, M, D, and E immunological test system. (a)Identification. An immunoglobulins A, G, M, D, and E immunological test system is a device that consists of the reagents used to measure by immunochemical techniques the immunoglobulins A, G, M, D, an E (serum antibodies) in serum. Measurement of these immunoglobulins aids in the diagnosis of abnormal protein metabolism and the body's lack of ability to resist infectious agents. (b)Classification. Class II (performance standards). [Code of Federal Regulations] [Title 21, Volume 8] [Revised as of April 1, 2013] [CITE: 21CFR866.5750] TITLE 21--FOOD AND DRUGS CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES SUBCHAPTER H--MEDICAL DEVICES PART 866 -- IMMUNOLOGY AND MICROBIOLOGY DEVICES Subpart F--Immunological Test Systems Sec. 866.5750 Radioallergosorbent (RAST) immunological test system. (a)Identification. A radioallergosorbent immunological test system is a device that consists of the reagents used to measure by immunochemical techniques the allergen antibodies (antibodies which cause an allergic reaction) specific for a given allergen. Measurement of specific allergen antibodies may aid in the diagnosis of asthma, allergies, and other pulmonary disorders. (b)Classification. Class II (performance standards).