Revision sheet - Science
advertisement

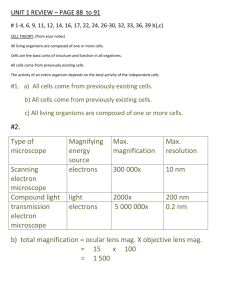
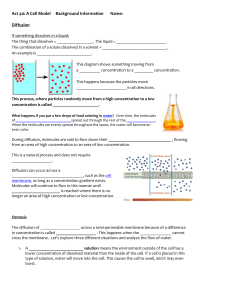
Last minute revision – Human Biological Science test 1 Cell structure and organelles Cells are the basic units of life. They contain tiny parts called organelles that have specific roles for life. Cell membrane allows transport in and out of the cell and provides a boundary from external fluid Mitochondria - the place where respiration takes place. High energy requiring cells will contain lots of mitochondria Endoplasmic reticulum is responsible for protein synthesis The nucleus contains DNA and controls the cell function. Structure of the cell membrane as it relates to transport of materials Cell membrane made of mainly lipids and proteins. Cell membrane is a phospholipids bilayer (2 layers) The lipid molecule contains a phosphate group = Phospholipid Cell membrane is differentially permeable – allows some molecules and restricts others Passive processes – don’t use energy from respiration, active processes – requires cells energy. 3 methods of transfer: Diffusion, carrier mediated and Vesicular Methods of transporting materials including diffusion, facilitated diffusion, osmosis, active transport, endocytosis and exocytosis Diffusion: Small molecules such as water and gases can passively diffuse through the membrane – high –low. Oxygen diffuses into the cell along th concentration gradient as the concentration inside the cell is low as the oxygen is continually being used by respiration. No limitation on rate – the larger the concentration difference the faster the rate of diffusion Osmosis is the diffusion of water –high conc of water (dilute solution) to a low conc of water (concentrated solution). Water can move through as it is small. Other substances such as salt and sugar cannot as they are large. As water moves into a cell osmotic pressure increases Carrier medicated transport also called Facilitated diffusion. There are special proteins in the membrane that bind to an ion or molecule and help it move across the membrane- such as glucose and amino acids. When the transported molecule binds to the carrier protein , the protein changes shape carrying the molecule to the opposite side of the cell membrane. Once all carrier molecules in use it cant go any faster Active transport –molecules move against the concentration gradient – requires energy. E.g. amino acids and certain ions that are already more concentrated in the cell can still be absorbed – energy for active transport comes from cellular respiration. Vesicular-Active process – move in and out of the cell in enclosed vesicles – exocytosis/endocytosis Endocytosis – cell surrounds some extracellular material , cell membrane folds and material enclosed – phagocytosis (cell eating) Pinocytosis (cell drinking) Exocytosis – contents of vesiucle pushed out. Membrane around vesicle fuses with cell membrane and contents are passed to the exterior of cell e.g. milk from breast, saliva Factors affecting exchange of materials including surface area to volume ratio, concentration gradients. All requirements of a cell must pass through cell membrane. Thus relationship between SA and vol is important. As a cell grows its ability to exchange enough materials to support its increasing volume diminishes. A large cell could not support itself as it would not have enough cell membrane surface to absorb all the nutrients required or remove the wastes. Therefore to function – cells need to be microscopic! Tiny!! Anabolic and catabolic reactions and organelles involved Anabolic – to build uses energy – e.g. Protein synthesis – In the Ribosomes Catabolic – to break down – releases energy in the mitochondria Most reactions take place in the cytoplasm of the cell Respiration is an example of a catabolic reaction that releases energy when molecules of ATP are broken down Respiration (aerobic and anaerobic); inputs, outputs and organelles involved Ceullar respiration – process by which molecules taken in as food as broken down in the cells using oxygen to release energy for the cells activities – 7 life processes Glucose+ oxygen carbon dioxide + water + energy Most of energy made is used to maintain body temperature. The remaining energy is used to form a compound called ATP Takes place in every cell of the body. The main food material utilized (used) is glucose Anaerobic respiration – without oxygen – breaks down glucose into pyruvate then lactic acid via Glycolysis . This releases enough energy to convert 2 molecules of ADP to ATP Anaerobic respiration takes place in the cytoplasm as this is where the enzymes are available for the reaction. Anaerobic respiration is important during vigorous physical activity e.g. in muscle cells to supply extra energy. This results in the accumulation of lactic acid in the muscles – pain and fatigue. Lactic acid is taken to the Liver where it can be re-combined with oxygen to form glucose and eventually glycogen Aerobic respiration uses oxygen – it breaks down 2 pyruvate molecules from glycolysis to carbon dioxide and water. This happens in the mitochondria 2 reactions allow this to occur – 1st = Krebs cycle, 2nd electron transport system – produce up to 36 molecules of ATP Enzyme function including reduction in activation energy, lock and key principle Biological catalysts that speed up chemical reactions by allowing them to occur at normal temperatures. Energy needed to get a reaction started = activation energy – enzymes reduced the activation energy needed to begin a reaction Also allow reactions to proceed at a rate that suits body requirements Enzymes are specific and made of proteins. Each enzymes will combine with one particular substrate. They have a shape and structure that allow them to fit together – Lock and Key The part of the enzyme that combines with the substrate is called the active site Factors that affect enzyme activity including pH, temperature, cofactors, co-enzymes. The higher the concentration of enzyme – faster the reaction The rate of most enzymes increase as temp increases but only in a limited range Above 45oC enzymes become denatured – inactive This is because they are proteins and the heat causes their shape to change The temp at which as enzyme works best = optimum temperature Enzymes are sensitive to pH Some enzymes need the help of co-factors such as ions or non-protein molecules Co-factors can change the shape of the active site which allow enzyme and substrate to combine