Properties of Matter Lab Purpose: Many forms of matter can be
advertisement

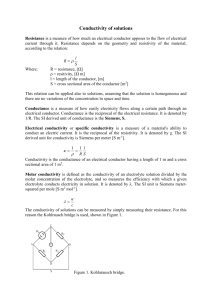
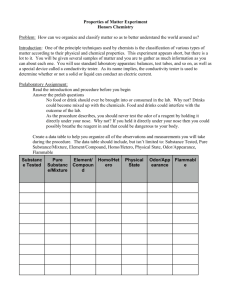
Properties of Matter Lab Purpose: Many forms of matter can be grouped together based on their shared properties. These properties include if they can conduct an electric current, are flammable, color, and ability to dissolve in water. Introduction: One of the principle techniques used by chemists is the classification of various types of matter according to their physical and chemical properties. This experiment appears short, but there is a lot to it. You will be given several samples of matter and you are to gather as much information as can about each one. You will use standard laboratory equipment: scoopulas, well plates, transfer pipets, and test tubes. You will also use a special device called a conductivity tester. As its name implies, the conductivity tester is used to determine whether or not a solid or a liquid can conduct an electric current. Its use will be demonstrated in class. Materials: Equipment Lab apron Goggles Conductivity tester Well plate Transfer pipets Universal indicator 100 ml Beaker Solids Steel wool (iron) Magnesium pieces Ammonium chloride Sucrose (table sugar) Sodium carbonate Copper sulfate crystals Sodium chloride Liquids Acetone Isopropanol (rubbing alcohol) Tap water Vinegar Household ammonia Safety: 1. Most of the chemicals can be toxic if ingested or inhaled. 2. Use the wafting technique to determine the odor of certain chemicals. Procedure: 1. Fill a 100mL beaker with about 50mL of water. 2. Place a small sample of each material into each well of your well plate. (Tip of a scoopula for a solid, 5-10 drops for a liquid) 3. Record the color, odor, and texture of each material. Be specific - examples include: shine, smooth, grainy, etc. 4. Add 5-10 drops of tap water to each material to see if it is soluble (dissolves) or insoluble (does not dissolve). *For a liquid: is soluble in water if water disappears into solution. If there is a clear indication that the 5. Place 2-3 drops of Universal Indicator to each well to see if the material is acidic or basic. Record the color and identify if it is acidic, neutral, or basic. 6. Place the conductivity tester into each sample. Wipe with a paper towel or tissue between samples. Record whether the material weakly, moderately, or strongly conducts an electric current. a. Weak conductor – only red light b. Moderate conductor – red light and weak green light c. Strong conductor – both red and green light 7. Place waste in the bucket located inside the hood. 8. Clean all well plates, beakers, and lab benches thoroughly before leaving the lab area. 9. The instructor will conduct the flammability tests as a demonstration. Record your observations in your data table. Data Table: Material Steel wool (iron) Magnesium pieces Ammonium chloride Sucrose (table sugar) Sodium carbonate Copper sulfate crystals Sodium chloride Color Odor Solubility Acid/Base Conductivity Flammability Acetone Isopropanol Tap water Vinegar Household ammonia Conclusion: Write a conclusion that answers the following questions: Based on your results, what materials can be grouped together based on their shared properties? What generalization can you make about the connection between solubility and the ability to conduct an electric current? What generalizations can you make about acidic and basic properties? What about flammability?