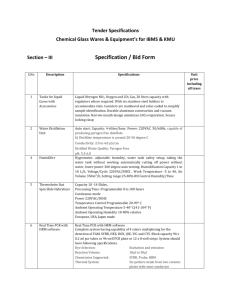
Supplementary Materials and Methods Subjects and sampling Fifty
advertisement

1 Supplementary Materials and Methods 2 Subjects and sampling 3 Fifty four obese Chinese adults (body mass index [BMI] > 26 kg/m 2, 31 females and 23 males) 4 and 54 sex- and age-matched lean control (18≤ BMI ≤23 kg/m2) subjects in this study were selected 5 from a case-control study in which over 500 pairs of overweight/obese and normal-weight control 6 people were recruited from November 2007 to January 2008 (Sun et al. 2010). In brief, the 108 7 volunteers were 35- to 54-year-old Chinese Han people who had lived in the urban area of Shanghai, 8 China, for at least 10 years. Individuals who were alcoholic, diabetic, with organic/infectious diseases, 9 with severe psychological disorders or physical disabilities, pregnant/lactating, or had diarrhea or 10 antibiotic treatment within 3 months prior to the recruitment were excluded. For each volunteer in this 11 study, the waist and hip circumference, waist-hip ratio (WHR), fasting glucose level, systolic and 12 diastolic blood pressure (SBP and DBP) were adopted from previously published study on over 500 13 pairs of overweight/obese and normal-weight control people (Sun et al. 2010), and re-calculated in the 14 context of this study. The volunteers were asked to keep their normal diets before and at the time of 15 sampling. Freshly passed feces were collected from each volunteer and stored at -80°C before analysis, 16 and fecal DNA was extracted using InviMag® Stool DNA Kit (Invitek GmbH, Berlin, Germany) in 17 accordance with the manufacturer’s instruction. The protocol of the study was approved by the 18 Institutional Review Board of Institute for Nutritional Sciences, Shanghai Institutes for Biological 19 Sciences, Chinese Academy of Sciences, and was performed in accordance with the Declaration of 20 Helsinki and the later amendments. Written informed consent was obtained from each volunteer before 21 the participation in the study. 1 22 23 24 Quantitative Real-time PCR Real-time PCR was performed on a DNA Engine Opticon 3 system (MJ Research, Waltham, MA, 25 USA). Primer pairs, FPR-2(5’-GGAGGAAGAAGGTCTTCGG-3’) and Fprau645R 26 (5’-AATTCCGCCTACCTCTGCACT-3’) (Ramirez-Farias et al. 2009), and Uni-1 (331F) 27 5’-TCCTACGGGAGGCAGCAGT-3’ and Uni-2 (797R) 28 5’-GGACTACCAGGGTATCTAATCCTGTT-3’ (Nadkarni et al. 2002) were used to quantify fecal F. 29 prausnitzii and total bacteria, respectively. 30 Each 25-μl reaction mixture contained 0.75U TaKaRa rTaq polymerase (Takara, Dalian, China), 31 12.5μl of 2×Sybmix (BioEasy SYBR Green I Real-time PCR Kit, Hangzhou, China), 25 pmol of each 32 primer, and extracted fecal DNA (40 ng and 20 ng for quantification of F. prausnitzii and total bacteria, 33 respectively). F. prausnitzii were quantified with the following program: 3 min at 95°C, 40 cycles of 30 34 s at 95°C, 30 s at 60°C, 30 s at 72°C, and 10 s at 83°C for fluorescence detection. Total bacterial were 35 quantified with the following program: 4 min at 95°C, 40 cycles of 15 s at 95°C, 1 min at 60°C, 5s at 36 80°C for fluorescence detection. To confirm the specificity of the PCR reaction, melting curve analysis 37 was performed after amplification by increasing the temperature at a rate of 0.5°C per 10 s from 60°C 38 to 95°C with continuous fluorescence monitoring. 39 The gene copy number of bacteria in feces was quantified using standard curves constructed from 40 known concentrations of plasmid DNA ranging from 1×108 to 1×102 copies/μl for F. prausnitzii and 41 1×109 to 1×103 copies/μl for total bacteria. 42 Each PCR reaction was performed in triplicate. The abundance of F. prausnitzii was calculated by 2 43 dividing the gene copy number of F. prausnitzii by that of total bacteria, and expressed as the 44 percentage of F. prausnitzii accounting to the total bacteria. 45 46 47 Statistical analysis Comparison between volunteer groups was performed with Student’s t test for data with normal 48 distribution and with Mann-Whitney U test for data without normal distribution. Spearman’s rank 49 correlation was performed to study the association between the abundance of F. prausnitzii and clinical 50 parameters. All statistical analysis was carried out with PAST software (Hammer et al. 2001), and P 51 values less than 0.05 were taken as statistically significant. 52 53 Supplementary References 54 Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for 55 56 57 Education and Data Analysis. Palaeontologia Electronica 4 (1):9 Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148 (Pt 1):257-266 58 Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P (2009) Effect of inulin on the 59 human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium 60 prausnitzii. Br J Nutr 101 (4):541-550 61 Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clement K, Hu FB, Lin X (2010) A 62 marker of endotoxemia is associated with obesity and related metabolic disorders in 63 apparently healthy Chinese. Diabetes Care 33 (9):1925-1932 3