WEEK 7 Feb. 19-22
advertisement
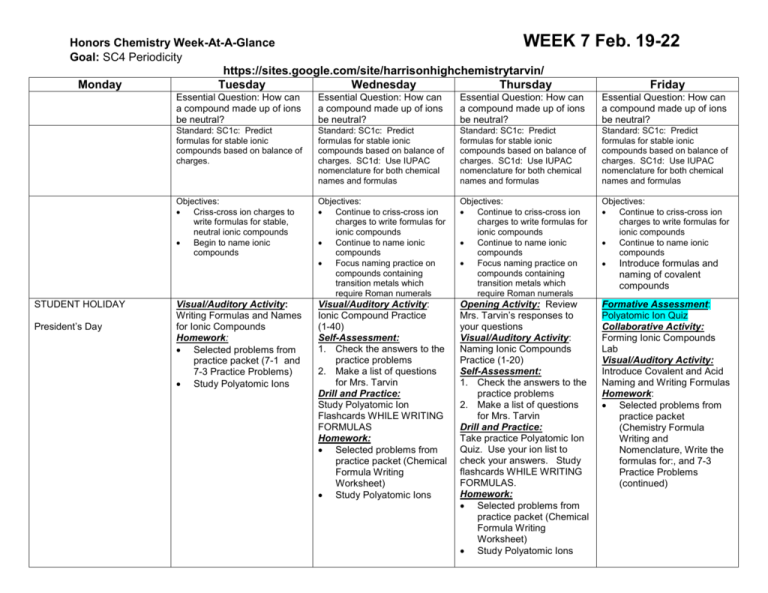
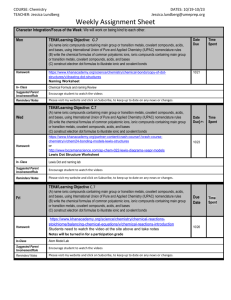
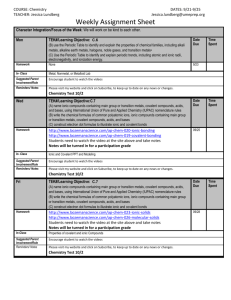
Honors Chemistry Week-At-A-Glance WEEK Goal: SC4 Periodicity https://sites.google.com/site/harrisonhighchemistrytarvin/ Monday Tuesday Wednesday Thursday STUDENT HOLIDAY President’s Day 7 Feb. 19-22 Friday Essential Question: How can a compound made up of ions be neutral? Essential Question: How can a compound made up of ions be neutral? Essential Question: How can a compound made up of ions be neutral? Essential Question: How can a compound made up of ions be neutral? Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas Objectives: Criss-cross ion charges to write formulas for stable, neutral ionic compounds Begin to name ionic compounds Objectives: Continue to criss-cross ion charges to write formulas for ionic compounds Continue to name ionic compounds Focus naming practice on compounds containing transition metals which require Roman numerals Objectives: Continue to criss-cross ion charges to write formulas for ionic compounds Continue to name ionic compounds Focus naming practice on compounds containing transition metals which require Roman numerals Objectives: Continue to criss-cross ion charges to write formulas for ionic compounds Continue to name ionic compounds Introduce formulas and Visual/Auditory Activity: Ionic Compound Practice (1-40) Self-Assessment: 1. Check the answers to the practice problems 2. Make a list of questions for Mrs. Tarvin Drill and Practice: Study Polyatomic Ion Flashcards WHILE WRITING FORMULAS Homework: Selected problems from practice packet (Chemical Formula Writing Worksheet) Study Polyatomic Ions Opening Activity: Review Mrs. Tarvin’s responses to your questions Visual/Auditory Activity: Naming Ionic Compounds Practice (1-20) Self-Assessment: 1. Check the answers to the practice problems 2. Make a list of questions for Mrs. Tarvin Drill and Practice: Take practice Polyatomic Ion Quiz. Use your ion list to check your answers. Study flashcards WHILE WRITING FORMULAS. Homework: Selected problems from practice packet (Chemical Formula Writing Worksheet) Study Polyatomic Ions Formative Assessment: Polyatomic Ion Quiz Collaborative Activity: Forming Ionic Compounds Lab Visual/Auditory Activity: Introduce Covalent and Acid Naming and Writing Formulas Homework: Selected problems from practice packet (Chemistry Formula Writing and Nomenclature, Write the formulas for:, and 7-3 Practice Problems (continued) Visual/Auditory Activity: Writing Formulas and Names for Ionic Compounds Homework: Selected problems from practice packet (7-1 and 7-3 Practice Problems) Study Polyatomic Ions naming of covalent compounds Honors Chemistry Week-At-A-Glance WEEK 8 Goal: SC1 Bonding https://sites.google.com/site/harrisonhighchemistrytarvin/ Monday Tuesday Wednesday Thursday Essential Question: How are compounds formed from only nonmetals? Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas SC3e Compare and contrast types of chemical bonds Objectives: Writing formulas and naming of covalent compounds Writing formulas and naming acids Introduce basic Lewis structures Apply electronegativity concepts to Lewis structures to predict polarity DUE: Forming Ionic Compounds Lab Report Formative Assessment: Ionic Naming and FormulaWriting Quiz Visual/Auditory Activity: Drawing Lewis Structures and predicting polarity Homework: Review all drawings from class Selected problems from practice packet (Lewis Structures, VSEPR, Polarity, IM Forces) Essential Question: How are compounds formed from only nonmetals? Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas SC3e Compare and contrast types of chemical bonds Objectives: Writing formulas and naming of covalent compounds Writing formulas and naming acids Draw advanced Lewis structures Apply electronegativity concepts to Lewis structures to predict polarity Formative Assessment: Covalent & Acid Naming and Formula-Writing Quiz Self-Assessment: Return Ionic Compound Quiz and go over Visual/Auditory Activity: Drawing advanced Lewis structures and predicting the polarity of the compound Visual/Auditory Activity: Intro VSEPR Bonding Theory Homework: Review all drawings from class Selected problems from practice packet (8-1 Practice Problems drawings ONLY) Feb. 25-March 1 Friday Essential Question: How are compounds formed from only nonmetals? Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas SC3e Compare and contrast types of chemical bonds Objectives: Use polarity to predict type of intermolecular forces Build models of covalent compounds to predict VSEPR geometry Essential Question: How are compounds formed from only nonmetals? Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas SC3e Compare and contrast types of chemical bonds Objectives: Use polarity to predict type of intermolecular forces Build models of covalent compounds to predict VSEPR geometry Essential Question: How are compounds formed from only nonmetals? Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas SC3e Compare and contrast types of chemical bonds Objectives: Review Visual/Auditory Activity: VSEPR Bonding Theory and Predicting the Intermolecular Forces within samples of the compound Kinesthetic Activity: Ball & Stick Modeling Lab Homework: Selected problems from practice packet (8-1 Practice Problems answer questions about drawings and More Fun with Lewis Structures) Prepare for Combined Quiz with “Review – Naming Chemical Compounds” DUE: Ball & Stick Modeling Lab DUE: Compare & Contrast Ionic and Covalent Compounds Lab Review Activity: Bonding Review Webquest and Sample Multiple Choice Questions Formative Assessment: Combined Ionic, Covalent, and Acid Quiz Kinesthetic Activity: Compare and contrast ionic and covalent compounds lab Homework: Begin Bonding Review Webquest and Sample Multiple Choice Questions via blog Honors Chemistry Week-At-A-Glance WEEK Goal: Reactions https://sites.google.com/site/harrisonhighchemistrytarvin/ Monday Tuesday Essential Question: What are the physical and chemical properties of ionic compounds? Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas SC3e Compare and contrast types of chemical bonds Essential Question: What is the difference between ionic and covalent compounds? Objectives: Assess mastery of standards Objectives: Assess mastery of standards UNIT FOUR LAB PRACTICAL UNIT FOUR TEST CUMULATIVE QUIZ Review sample questions for tomorrow’s test. Standard: SC1c: Predict formulas for stable ionic compounds based on balance of charges. SC1d: Use IUPAC nomenclature for both chemical names and formulas SC3e Compare and contrast types of chemical bonds 9 March 4-8