hwsol2012_04
advertisement

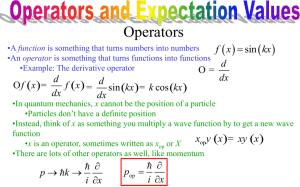
Physics 249 Homework 4 Due Oct 5th 2 2 1) A particle has a wave function given by 𝜓(𝑥) = 𝐴𝑒 −𝑥 /2𝐿 and energy. 𝐸 = ℏ2 /2𝑚𝐿2 . a) Find the potential energy as a function of x. b) What is the classical potential that has this dependence. To find the potential insert the wave function into the Schrodinger equation. ℏ2 𝜕 2 𝜓(𝑥) 𝐸𝜓(𝑥) = − + V(x)𝜓(𝑥) 2𝑚 𝜕𝑥 2 ℏ2 ℏ2 𝜕 1 𝜓(𝑥) = − (− 2 𝑥𝜓(𝑥)) + V(x)𝜓(𝑥) 2 2𝑚𝐿 2𝑚 𝜕𝑥 𝐿 ℏ2 ℏ2 1 1 𝜓(𝑥) = − (− 2 𝜓(𝑥) + 4 𝑥 2 𝜓(𝑥)) + V(x)𝜓(𝑥) 2 2𝑚𝐿 2𝑚 𝐿 𝐿 V(x)𝜓(𝑥) = V(x) = ℏ2 1 2 𝑥 𝜓(𝑥) 2𝑚 𝐿4 ℏ2 1 2 𝑥 2𝑚 𝐿4 The harmonic oscillator has this dependence. 2) Normalize the wave function in problem 1 𝜓(𝑥) = 𝐴𝑒 −𝑥 2 /2𝐿2 ∞ ∞ ∫ 𝜓 ∗ (𝑥)𝜓(𝑥)𝑑𝑥 = ∫ 𝐴2 𝑒 −𝑥 −∞ 2 /𝐿2 𝑑𝑥 = 1 −∞ Using from any table on integrals and noting that the function is symmetric around zero. I used http://en.wikipedia.org/wiki/List_of_integrals_of_exponential_functions ∞ 2 ∫ 𝑒 −𝑎𝑥 𝑑𝑥 = 0 1 𝜋 √ 2 𝑎 4 1 𝐴2 √𝜋𝐿2 = 1, 𝐴 = √𝜋𝐿2 4 𝜓(𝑥) = √ 1 −𝑥 2 /2𝐿2 𝑒 𝜋𝐿2 3) a) Normalize the wave function 𝜓 = 𝐴𝑒 𝑖(𝑘𝑥−𝜔𝑡) between x= –a and a. b) Can the wave function be normalized between + and – infinity? c) Explain how what the answer to part b) says about why a simple wave is an unphysical solution to explain light. 𝑎 𝑎 ∫ 𝜓 ∗ (𝑥)𝜓(𝑥)𝑑𝑥 ∫ 𝐴2 𝑑𝑥 = 2 𝐴2 𝑎 = 1 −𝑎 −𝑎 1 𝐴=√ 2𝑎 The integral of the wave function would be infinite between negative and positive infinity. Therefore, the wave function cannot be normalized over all space. This function, usually called the plane wave function, is not normalizable and thus cannot represent a physical solution for light. This should be clear because you light “wave” cannot extend over all space. For instance, it would not be quantized into particles and it would have to have infinite energy. A wave packet can be normalized and represents a solution to the wave equation that accurately describes the properties of light. 4) A particle with the mass of the electron is in the ground state of an infinite potential of length L. a) What value of L gives an energy magnitude equal to that of the hydrogen atom. Compare that to the Bohr radius. b) Is a particle in a 1D box a good model for the electrons in an atom? (1240𝑀𝑒𝑉𝑓𝑚)2 𝜋 2 ℏ2 ℎ2 𝑐 2 𝐸=1 = = = 13.6𝑥10−6 𝑀𝑒𝑉 2𝑚𝐿2 8𝑚𝑐 2 𝐿2 8(0.511𝑀𝑒𝑉)𝐿2 2 L = 166000 fm = 0.166 nm The Bohr radium is 5.29x10-2 nm No the particle in a box is not a good model. The atom should be solved in 3 dimensions and using a 1/r potential which gives a substantially different functional dependence on the quantum number. These differences will change the solution substantially 5) A particle is confined in an infinite potential of length L. a) Use the uncertainty principle to estimate the smallest possible energy of the particle. Note that the particle is “uncertain “ in momentum since it is bouncing back an forth and may have a +- momentum at any given moment and uncertain in position within the size of the box. b) Compare this to the energy of the ground state energy calculated using the Schrodinger equation. The uncertainty represents the minimum delta p the particle has. The particle is bouncing back and forth in the box it has a minimum uncertainty of 2p, from –p to p, and an approximate rms uncertainty of p. The maximum delta x is L and the rms is approximately 0.5L The minimum energy can be calculated from this number. p = Δ𝑝 = ℏ ℏ = 2Δ𝑥 𝐿 𝑝2 ℏ2 𝐸= = 2𝑚 2𝑚𝐿2 𝜋 2 ℏ2 𝜋 2 ℏ2 𝐸=1 = 2𝑚𝐿2 2𝑚𝐿2 2 About a factor of 10 difference, but with the correct dependence, which is much closer than in the previous problem. In this case the uncertainty principle in 1D can be legitimately compared to the solution in 1D for the infinite potential since the functional dependence of the uncertainty on L or p is understood. To more realistically solve the problem we should calculate the expectation values for x and p.