Abstract - Chris Lewis` Portfolio
advertisement

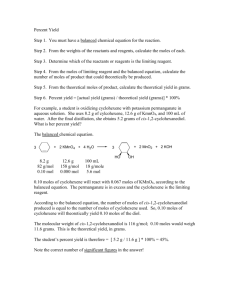
Studying the Temperature Affect on Yield and Rate of the Epoxidation of Cyclohexene By Chris Lewis A Research Paper Submitted in Partial Fulfillment of the Requirements for the Bachelor of Science Degree in Biochemistry Dr. David Oostendorp, PHD Loras College May, 2012 Depertment of Molecular and Life Science Loras College Dubuque, IA Author: Lewis, Chris E. Title: Studying the Temperature Effect on Yield and Rate of the Epoxidation of Cyclohexene Degree/Major: Biochemistry Research Advisor: Dr. David Oostendorp, PHD Month/Year: May, 2012 Number of Pages: 5 Abstract A study reviewing the effects that temperature has on the epoxidation of cyclohexene using mCPBA. The concentrations of the reaction were experienced with 1.2 equivalents of mCPBA in methylene chloride. The reactions were measured at room temperature, 5°C, and 40°C. Results showed that rate of reaction at 5°C was 20 minutes faster than the reaction at room temperature; as well as producing a yield with only 6 % difference. However at 40°C, the product formed was not an epoxide, but an alcohol group. This was most likely due to the mCPBA forming the epoxide then breaking it back open, forming the alcohol group. Introduction Epoxide molecules are three membered rings that contain two carbon atoms bonded to one oxygen molecule. Reacting peroxy acids with alkenes, usually in methylene chloride solvent, form the epoxides. Epoxides are typically useful because they are used in the polymerization process. The epoxide can be broken open and linked together to form polymer chains. Epoxides are also used in adhesives and protective coatings. The epoxidation reaction is typically carried out at room temperature for a half an hour. The rate and times vary depending on the different alkenes used; particularly what types of functional groups are on the alkene. Research shows that the reaction rate is quicker depending on the amount of electron donating groups on the alkene, as well as the amount of electron withdrawing groups on the peroxy acid (Kim, et. al., 1998). These groups will make it easier for the peroxy acid easier to attack the pi bond on the alkene. These functional groups can also cause steric hindrance, blocking the acid from the pi bond and producing low yields (Kim, et. al., 1998). Epoxides have also been reacted at low temperatures in order to push certain stereoisomers (Palucki, et. al., 1994). Specifically, a study performed at Harvard University measured the epoxidation reaction of styrene at -78 ° C in order to try to push just the trans isomer (Palucki, et al., 1994). Epoxides have also been studied under biological systems. Although they have not been found as acting molecules in these systems, they have been determined to be reactive intermediates (Iyer, et. al., 1993). This information was determined by the structures of DNA abducts, along with multiple bioreceptors (Iyer, et al., 1993). This research discusses the importance of the electron donating groups affecting the rate of reaction on the epoxidation reaction. My interest was to study the effect that temperature would have on the rate and yield. I to studied both the cold and warm effect of temperature. Since this reaction is run at room temperature, it was particularly simple to manipulate the temperature. So in order to isolate temperature as my dependant variable, I used cyclohexene, a 6 membered ring with no alternating functional groups on it. Since there are no electron donating groups on the alkene, I expected to experience good yields due to the lack of steric hindrance. Materials and Methods The initial reaction was run at room temperature and used as a baseline to compare the temperature manipulation reactions to. 1.03g (1.2 equivalents) of meta-chloroperoxybenzoic acid (mCPBA) was dissolved in methylene chloride, and then mixed with .405g of cyclohexene. This reaction was run at room temperature for 30 minutes with continuous stirring. As the reaction ran, the acid precipitated out of the solvent and was filtered out. The solution was then put through a simple distillation in order to distill away the methylene chloride. In order to identify the product, the molecule was then placed in an Infrared Spectrometer. During the next phase of the experiment I need to manipulate the temperature. The concentrations and the peroxy acid were the same as in the initial reaction. The reaction was run in an ice bath kept at 5° C, and the time took for the mCPBA to precipitate out was measured. The reaction was then filtered and distilled in the same way as the initial reaction. The product was identified through infrared spectroscopy and weighed for a yield. This reaction was repeated and the results were averaged. The same steps were taken when conducting the warm temperature manipulation. The only difference was that the reaction was run under a hot water bath that was held constant at 40° C. The product was then purified and identified through infrared spectroscopy. This manipulation was repeated and the results were averaged. One limitation of this manipulation is that the solvent, methylene chloride, boils at around 40° C, so it was tough to keep the solvent in the solution with out it boiling away from the reaction. Results After the base line reaction was finished the IR identified that the epoxide was formed onto the cyclohexene ring forming epoxycyclohexane. The reaction gave a 96% yield and took 30 minutes to complete. The reaction performed at 5° C took between 5-10 minutes to precipitate the reacting acid out of the solution. This reaction gave back an average yield of 90%. Fairly similar with the reaction performed at room temperature. The IR also confirmed that the epoxide had formed onto the cyclohexene. The reaction at 40° C, however, did not have as good results. The mCPBA did not precipitate out of the reaction mixture at that temperature, even when the reaction was run for 30-60 minutes. The reaction did however precipitate when the mixture was cooled to room temperature. The IR of the product did not show that an epoxide was formed, instead the reading showed and alcohol product. So no yield was taken due to no product was formed. Discussion After concluding the experiments and calculating the results I have concluded that temperature did have an effect on the rate, and the yield of the reaction. While the room temperature experiment did produce the best yield, it did not have the best rate. The reaction performed at 5° C did react 20 minutes faster than the reaction performed at room temperature. This may have happened because as the temperature dropped the solution became super-saturated and in return some of the acid precipitated out of the mixture. These results do comply with the research performed on styrene at -76°C (Palucki, et. al., 1994). Although, the reaction was not performed at this extreme of a temperature, but this was due to a lack of equipment. The reaction performed at 40°C did not give back the expecting results. It was very tough to get the reaction mixture to precipitate the reacting mCPBA out of the mixture at that temperature. The solution would remain clear and show no sign of precipitation. This could be due to the heat raising the saturation point and keeping the acid in the reaction, because as soon as the reaction cooled to room temperature the acid precipitated out. The IR spectroscopy reading of the product that was obtained did not, however, show that epoxycyclohexane was formed. Instead of the intended epoxide, an alcohol group was formed. Since the mCPBA never precipitated out of the solution, it is logical to assume that after the acid reacted to form the epoxide it then broke open the ring placing the alcohol group on one of the two carbons. An issue that I found during the warm temperature manipulation is that methylene chloride boils at just over 40° C, so it was tough to research many temperatures over room temperature. In conclusion the temperature did have a effect on the yield and temperature of epoxidation of cyclohexene. Although the yields were similar when exploring the room temperature and 5° C experiment, the warm temperature manipulation did not produce the epoxide. So the yield was significantly effected at 40°C. The rate was also affected. The cold temperature manipulation (5°C) took on average 20 minutes less time to react in comparison to the room temperature experiment. With that being said, the reaction performed at room temperature held the best yield, and even thought it took 20 more minutes it was an easier experiment to control. References Kim, C.,;Traylor, T.; Perrin, C. Mcpba epoxidation of alkenes: Reinvestigation of correlation between rate and ionization potential. 1998. Journal of the American Chemical Society, 129(37), 9513-9516. Palucki, M.; Pospisil P. J.; Zhang W.; Jacobsen E. N.; High enantioselictive, lowtemperature epoxidation of styrene. 1994. Journal of the American Chemical Society. 116. 9333-9334 Iyer, R.; Harris,T. Preparation of aflatoxin B 8,9 epoxide using metachloroperoxybenzoic acid.1993. Chem. Res. Toxical, 6(3). 313-316.