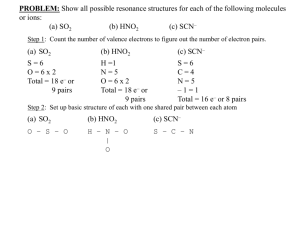
exercise
advertisement

1. Limestone (CaCO3) is used in a throwaway limestone scrubbing system for a powerplant burning coal containing 3.60% sulfur and 7.70% ash. 90 percent of the SO2 must be removed in order to meet the emission standards. Determine (a) the amount of sludge that will be produced per ton of coal if the sludge is 60% water and 40% CaSO4 . 2H2O, plus ash. Hints: * Assume that all SO2 is trapped as CaSO4 . 2H2O * Remember three things when you write the overall reaction: (1) some extra oxidation is generally necessary to convert the calcium sulfite to calcium sulfate (2) you prepare a slurry of limestone, i.e. water is also among the reactants.(3) the carbon in the limestone is oxidized to CO2. 2. A waste stream of 500 m3/s flow rate and containing 0.05% SO2 is to be treated with 85% removal efficiency in a wet scrubber using water. Determine the volumetric flow rate needed for this removal. Hanry’s law constant for SO2 = 50 atm 3. . a. Henry’s law constant for O2 in water at 20ºC is 40100 atm. Henry’s law constant for H2S in water at 20ºC is 453 atm. Which one is more soluble in water? b. We want to treat a natural gas stream for reducing the H2S content in it before it is supplied to the end-users. We are planning to use a simple absorber by using water scrubbing reagent at atmospheric pressure. The H2S content is 10 ppm while the permitted H2S gas concentration in the natural gas is 500 ppb. What is the Liquid/Gas ratio needed fort the treatment? xi = Pyi*/Hi 4. High sulfur amount in hydrocarbon fuels is a problem. Stationary sources such as oil fired power plants have control units to capture SO2 formed. On the other hand, mobile sources have no control units to reduce SO2. What is the best solution to reduce amount of SO2 emitted by mobile sources. Explain briefly and if needed write down the reaction. 5. A No.5 fuel oil having a maximum sulfur content of 2.0 % and heating value of 148000 Btu/gal is being considered as a fuel for an industrial boiler. If the density of the oil is 0.953 g/ml, what is the minimum efficiency required of a sulfur dioxide scrubber system in order for the plant to meet an emission standard of 0.8 lb/106 Btu? (S=32, O=16 g/mol) 6. a) The feed gas to a sulfuric acid plant has 7.8% SO2, 10.8% O2 and 81.4% N2. It is brought to chemical equilibrium over a catalyst at 500ºC, at which K ySO3 ( ySO2 )( yO2 )0.5 What fraction of the SO2 is converted to SO3? 85 b) Assume that a single absorption sulfuric acid plant recovers 98% of its SO2 as acid, and a double absorption plant recovers 99.7%. What fraction of the SO2 passing from the first absorber must be captured by the final conversion and absorption? c) If the final catalyst bed in part b operates at 420ºC, at which the equilibrium constant K=300, what fraction of the equilibrium constant is this? (Use the inlet composition in part a)