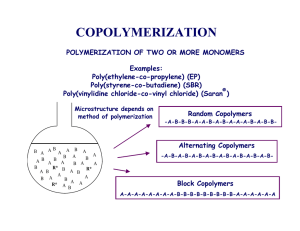
pubdoc_2_2446_775
advertisement
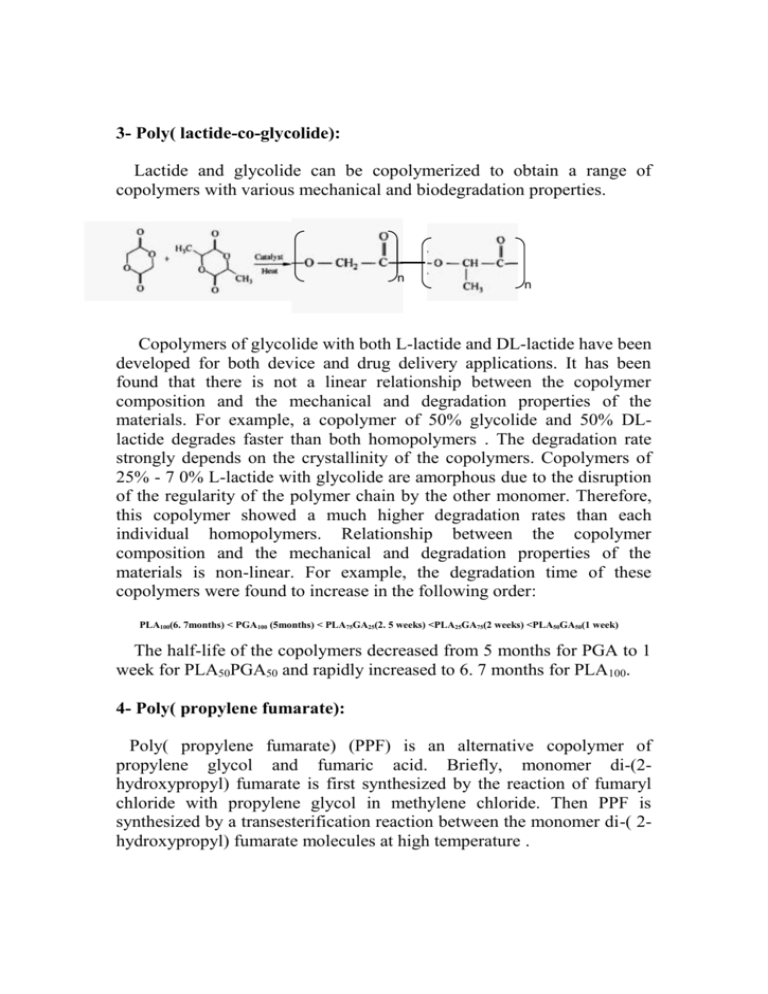
3- Poly( lactide-co-glycolide): Lactide and glycolide can be copolymerized to obtain a range of copolymers with various mechanical and biodegradation properties. n n n Copolymers of glycolide with both L-lactide and DL-lactide have been developed for both device and drug delivery applications. It has been found that there is not a linear relationship between the copolymer composition and the mechanical and degradation properties of the materials. For example, a copolymer of 50% glycolide and 50% DLlactide degrades faster than both homopolymers . The degradation rate strongly depends on the crystallinity of the copolymers. Copolymers of 25% - 7 0% L-lactide with glycolide are amorphous due to the disruption of the regularity of the polymer chain by the other monomer. Therefore, this copolymer showed a much higher degradation rates than each individual homopolymers. Relationship between the copolymer composition and the mechanical and degradation properties of the materials is non-linear. For example, the degradation time of these copolymers were found to increase in the following order: PLA100(6. 7months) < PGA100 (5months) < PLA75GA25(2. 5 weeks) <PLA25GA75(2 weeks) <PLA50GA50(1 week) The half-life of the copolymers decreased from 5 months for PGA to 1 week for PLA50PGA50 and rapidly increased to 6. 7 months for PLA100. 4- Poly( propylene fumarate): Poly( propylene fumarate) (PPF) is an alternative copolymer of propylene glycol and fumaric acid. Briefly, monomer di-(2hydroxypropyl) fumarate is first synthesized by the reaction of fumaryl chloride with propylene glycol in methylene chloride. Then PPF is synthesized by a transesterification reaction between the monomer di-( 2hydroxypropyl) fumarate molecules at high temperature . PPF has ester linkages in its structure. Therefore, PPF can be hydrolyzed through a hydrolysis mechanism. The structure units propylene glycol fumaric acid can be excreted via normal metabolic pathways. Fumaric acid is a component of Krebs cycle, and propylene glycol has been used as an intravenous fluid component. This polymer was developed mainly as injectable bone cement. The main advantage of this unsaturated polymer is the ability to cure the material in vivo, thereby filling the skeleton defect of any shape or size with minimal surgical intervention. The unsaturated double bond in its structure unit makes it possible to be crosslinked through radical polymerization. PPF can be cured or crosslinked using a vinyl monomer, with a radical initiating system such as benzoyl peroxide/dimethyl toluidine. Changing the PPF to vinyl monomer ratio can change the ultimate mechanical properties and the degradation rate of cured polymer. In vivo studies have demonstrated that NVP cross-linked PPF is a biocompatible biodegradable polymer. There is significant bone ingrowth into the polymer at 5 weeks post-implantation in a rat proximal tibia model study. 5-Polyamino Acid: The idea of using polyamino acid is that the peptide linkage can be broken down enzymatically. The degradation products then would be amino acids and polypeptide, which should be utilized by the body.