Lab Assignment #1 – Cobalt Equilibrium ( /40 )
advertisement
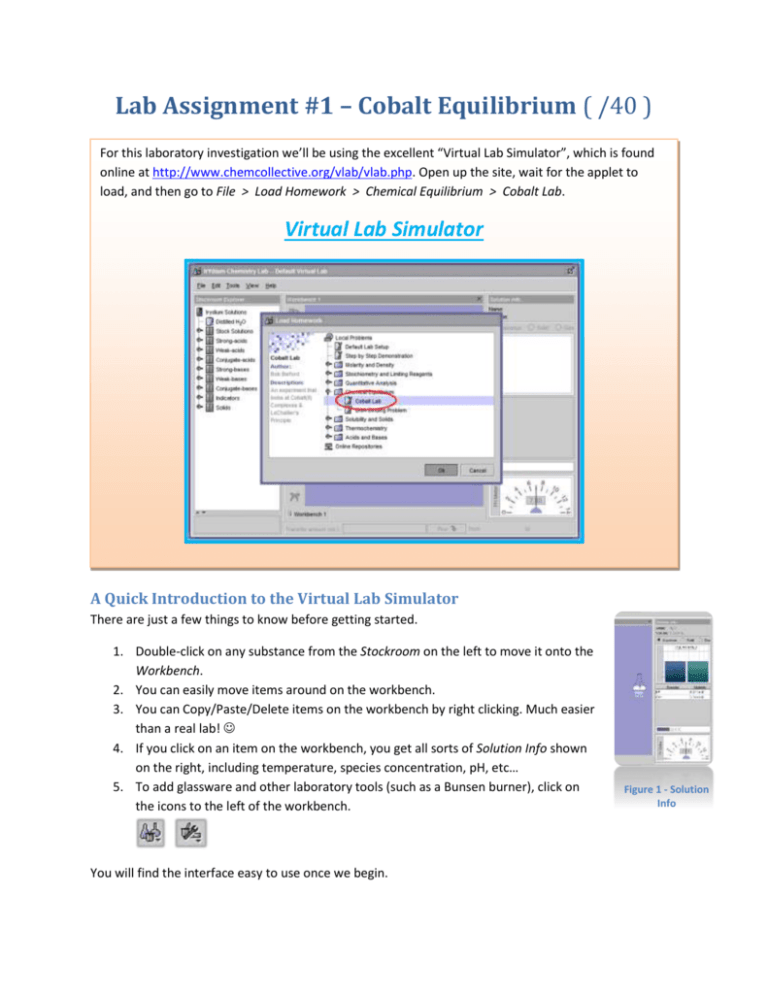
Lab Assignment #1 – Cobalt Equilibrium ( /40 ) For this laboratory investigation we’ll be using the excellent “Virtual Lab Simulator”, which is found online at http://www.chemcollective.org/vlab/vlab.php. Open up the site, wait for the applet to load, and then go to File > Load Homework > Chemical Equilibrium > Cobalt Lab. Virtual Lab Simulator A Quick Introduction to the Virtual Lab Simulator There are just a few things to know before getting started. 1. Double-click on any substance from the Stockroom on the left to move it onto the Workbench. 2. You can easily move items around on the workbench. 3. You can Copy/Paste/Delete items on the workbench by right clicking. Much easier than a real lab! 4. If you click on an item on the workbench, you get all sorts of Solution Info shown on the right, including temperature, species concentration, pH, etc… 5. To add glassware and other laboratory tools (such as a Bunsen burner), click on the icons to the left of the workbench. You will find the interface easy to use once we begin. Figure 1 - Solution Info Objective In this investigation, predictions using Le Chatelier’s principle will be tested through the observation of the effects of concentration and temperature stressors applied to an equilibrium system of various cobalt(II) complexes. Background Cobalt(II) does not exist in aqueous solution as a free ion, such as Co2+, but forms a complex ion where 6 water molecules attach to it. This results in the pink complex ion Co(H2O)6+2. In the presence of Cl- ions, a different complex forms, the blue CoCl4-2 complex ion. We can use their different colors to indicate the equilibrium concentrations for the following reaction: Co(H2O)6+2(aq) + 4Cl-(aq) pink CoCl4-2 (aq) + 6H2O(l) blue In this simulation you not only observe the equilibrium concentrations through their colors, but also directly read their concentrations (in the Solution Info panel on the right). Predictions /4 You will make a number of predictions before doing the investigation yourself. At the end we will revisit the predictions to see if you (and Le Chatelier!) were correct. Highlight or underline your answer, either Reactants or Products. 1.) Which direction will the system shift if HCl is added? 2.) Which direction will the system shift if the solution is diluted? 3.) Which direction will the system shift if AgNO3 is added? Reactants or Products Reactants or Products Reactants or Products Hint: You may need to consult your Table of Solubilities. 4.) Will the Keq value change as a result of the above changes? Assignment /14 Practical laboratory instructions are in bold. Questions you need to answer (you can do so on this document) are in green. 1. Add a stock solution of 1M CoCl2 to the workbench from the stockroom. Place a 250mL Erlenmeyer flask on the workbench. Transfer 50mL of the stock solution into the empty flask. (Drag stock solution onto the flask, and type in the transfer amount) Write down a few observations. What is the colour of the cobalt solution? What is the Keq value of this equilibrium system at room temperature (25°C)? First, determine the Keq expression. Next, record the equilibrium concentrations of the appropriate species in the solution (as seen in the Solution Info panel). The colour of the cobalt solution remains red. 2. Add a stock solution of 12M HCl from the stockroom to the workbench. Transfer 10mL of 12M HCl to your cobalt solution. Record your observations. Note any colour changes. Is there a temperature change? Once the system has reached a stable equilibrium and stable room temperature, record the solution concentrations and determine the Keq value. 3. Add a stock solution of 6M AgNO3 from the stockroom to the workbench. Transfer 10mL of 6M AgNO3 to your cobalt solution. Record your observations. Note any colour changes. Is there a temperature change? Anything else? Once the system has reached a stable equilibrium and stable room temperature, record appropriate species concentrations and determine the Keq value. 4. Let’s look more specifically at the effects of temperature. Right-click on your cobalt solution, and click on “Thermal Properties”. Insulate the flask from surroundings, and change the temperature to 0°C. Record your observations. Record species concentrations and determine the Keq value for the system at this temperature. 5. Right-click on your cobalt solution, and click on “Thermal Properties”. Insulate the flask from surroundings, and change the temperature to 100°C. Record your observations. Record species concentrations and determine the Keq value for the system at this temperature. 6. Quick Thinking: What could you do to shift the reaction heavily towards the products, to get a bright blue solution? Record your steps below and note and observations. Were you successful in shifting the system towards products to get a bright blue solution? 7. Lastly, we will consider the effects of dilution. Place an empty 500mL Erlenmeyer flask on the workbench. Right-click/filter the solid from your blue cobalt solution. Transfer 50mL of your cobalt solution to the 500mL Erlenmeyer flask. Place a solution of 100mL H2O from the stockroom on the workbench. Transfer the H2O to your 50mL cobalt solution. (You can transfer even more to see the effect.) Record your observations. Wait for the solution to return to 25°C and calculate the Keq value. Questions & Answers /18 1. How did the equilibrium system shift when HCl was added? What qualitative observations support this? Did this match your prediction? Explain the shift using Le Chatelier’s principle. 2. How did the equilibrium system shift when AgNO3 was added? What qualitative observations support this? Did this match your prediction? Explain using Le Chatelier’s principle. 3. How did the equilibrium system shift when it was diluted? What qualitative observations support this? Did this match your prediction? Explain using Le Chatelier’s principle. 4. Was the reversible reaction exothermic or endothermic? How do you know? Explain using Le Chatelier’s principle. (*Please note that reversible is not the same as reverse. That a reaction is reversible is a condition for any equilibrium.) 5. Did the Keq value for the equilibrium system exhibit any reasonable change as a result of the stresses you applied? If so, which stresses caused a change in the Keq value? Conclusion /4 Summarize the most important points of your results in one paragraph. How did your predictions hold up? What did you learn? A good conclusion always mentions the method that was used to arrive at that conclusion.








