Chris Leverington - Teachers in Industry
advertisement
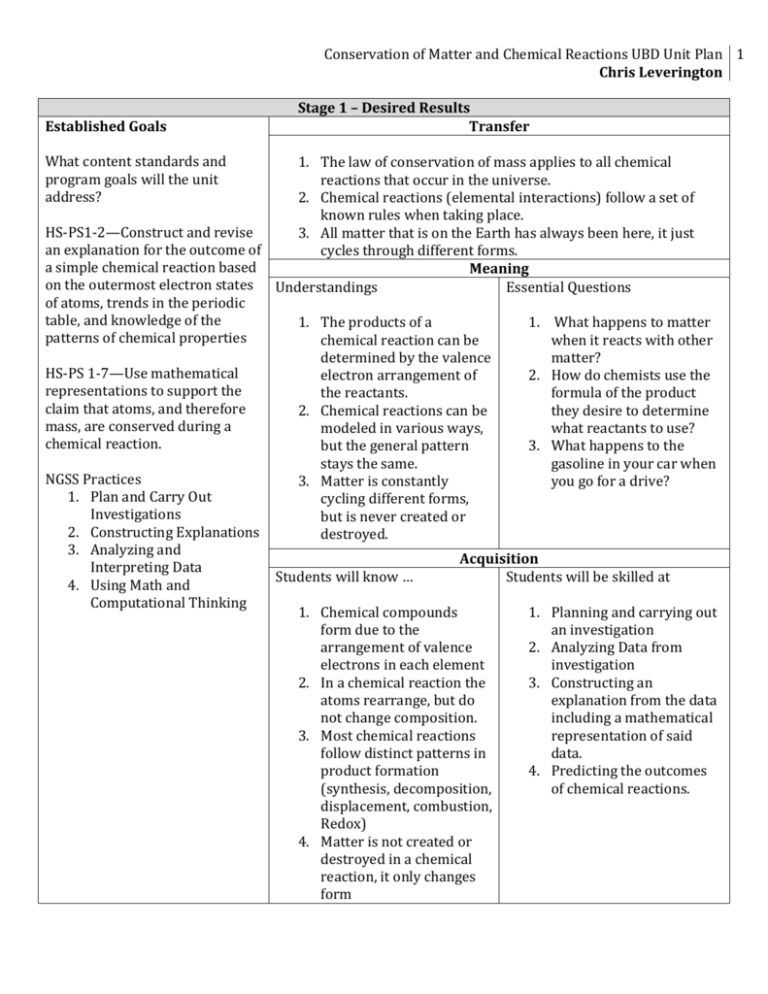
Conservation of Matter and Chemical Reactions UBD Unit Plan 1 Chris Leverington Established Goals What content standards and program goals will the unit address? Stage 1 – Desired Results Transfer 1. The law of conservation of mass applies to all chemical reactions that occur in the universe. 2. Chemical reactions (elemental interactions) follow a set of known rules when taking place. HS-PS1-2—Construct and revise 3. All matter that is on the Earth has always been here, it just an explanation for the outcome of cycles through different forms. a simple chemical reaction based Meaning on the outermost electron states Understandings Essential Questions of atoms, trends in the periodic table, and knowledge of the 1. The products of a 1. What happens to matter patterns of chemical properties chemical reaction can be when it reacts with other determined by the valence matter? HS-PS 1-7—Use mathematical electron arrangement of 2. How do chemists use the representations to support the the reactants. formula of the product claim that atoms, and therefore 2. Chemical reactions can be they desire to determine mass, are conserved during a modeled in various ways, what reactants to use? chemical reaction. but the general pattern 3. What happens to the stays the same. gasoline in your car when NGSS Practices 3. Matter is constantly you go for a drive? 1. Plan and Carry Out cycling different forms, Investigations but is never created or 2. Constructing Explanations destroyed. 3. Analyzing and Acquisition Interpreting Data Students will know … Students will be skilled at 4. Using Math and Computational Thinking 1. Chemical compounds 1. Planning and carrying out form due to the an investigation arrangement of valence 2. Analyzing Data from electrons in each element investigation 2. In a chemical reaction the 3. Constructing an atoms rearrange, but do explanation from the data not change composition. including a mathematical 3. Most chemical reactions representation of said follow distinct patterns in data. product formation 4. Predicting the outcomes (synthesis, decomposition, of chemical reactions. displacement, combustion, Redox) 4. Matter is not created or destroyed in a chemical reaction, it only changes form 2 CHRIS LEVERINGTON Stage 2 - Assessments Performance Task (in GRASPS format) [A][S]The biology students at Williams Field High School are having difficulty seeing how the law of conservation of matter applies to biological processes within the cell. [G]Your task is to provide the biology students with an explanation of the law of conservation of mass and demonstrate how mass is conserved in either the process of photosynthesis or in the process of cellular respiration. [R]As the experts in the field of matter conservation you will be teaching the biology students that the law of conservation of matter applies in all situations. You will research the chemical reactions that occur in the process you choose and determine how matter is conserved in that process. [P]You will create a poster, power point demonstration, or video to demonstrate the following information to the biology students: 1) An explanation of the Law of Conservation of Matter 2) A detailed drawing of the process that you chose (Photosynthesis or Respiration) including all intermediate steps and electron carriers. 3) A complete balanced equation of the reaction, including ATP, NAD+,NADH, etc. 4) A statement explaining how matter was conserved in this process. [S-2]Grading will be completed according to the attached rubric. Items that will be considered are accuracy of information in parts 1-3, your ability to accurately explain how matter was conserved in the process, neatness and appeal of your poster, and individual effort toward group success. Other Evidence: (quizzes, tests, prompts, work samples, labs, etc.) Students will complete worksheets regarding types of chemical reactions, electron movement in chemical reactions, predicting products of chemical reactions, balancing equations, and conservation of matter. Students will complete the “Law of Conservation of Matter Inquiry Lab,” the “Precipitate Lab,” and the “Types of Reactions Lab” Students will complete a quiz on types of reactions and balancing equations. Students will complete district unit test covering the Law of Conservation and Chemical Reactions. Student Self-Assessment and Reflection Students will self-assess and reflect using the following self assessment rubric. Conservation of Matter and Chemical Reactions UBD Unit Plan 3 Chris Leverington Stage 3 – Learning Plan Alka-Seltzer/Water demonstration—Demonstration will be used to pre-assess students understanding of the Law of Conservation of Matter (H) Discuss Performance Task Project (W) Direct Instruction on the Law of Conservation of Matter (E) Students will complete “Law of Conservation of Matter Inquiry Lab”(E) Discuss Determining Products of Reactions using Valence Electron Arrangement (W,E) Review Octet Rule and Lewis Structures Group work on determining products using electron arrangement (E, R) Direct Instruction on Balancing Equations (W, E) o Group work on balancing with manipulatives (E-2, O) o Worksheets on Balancing Equations (E-2) o Balancing Equations Race (E-2, R, T) Quiz over Law, Determining Products, and Balancing (E-2) Introduce Types of Reactions (W) o Students will carry out Precipitate lab (H) o Students will partner read Chapter 5, section 2 and draw conclusions regarding the types of reactions. (E) o Direct instruction on reaction types (E) o Students will be given manipulatives containing various reactants and products of chem reactions. Based on the reactants students will classify the reactions by type and determine the products for the reaction. (R, T, E-2, O) o Worksheets on types of reactions (E-2) o Students will complete the “Types of Reactions” Lab. (E, E-2, O) District Unit Exam on Law of Conservation of Matter and Reactions (E-2, T) Performance Task: o Students will readdress the performance task scenario. Students will research the reactions in the biological process chosen and determine the balanced equation and explanation of how it shows conservation of matter. (E-2, R, T, O) Create Poster and present to classes (E-2, R,T, O) Pre-Assessments What pre-assessments will you use to check student’s prior knowledge, skill levels, and potential misconceptions? Students will be given a preassessment to measure their understanding of the law of conservation of mass and chemical reactions. Students will also be given a short balancing equations preassessment. Progress Monitoring • How will you monitor students’ progress toward acquisition, meaning, and transfer, during lesson events? I will monitor their progress by checking their homework assignments, quiz scores. I will also monitor their progress via conversations and the student’s weekly self assessments. • What are potential rough spots and student misunderstandings? I believe the biggest area for confusion will be in predicting products. My experience in the past is that students struggle with this aspect of chemical reactions. I have never taught to look at valence electrons in this process before, so that might help. • How will students get the feedback they need Teacher will give formal feedback on assignments and quizzes. Teacher will provide informal feedback in one on one discussion based on weekly self-assessments. Students will also receive feedback from their peers during in-class learning activities. • Are all three types of goals (acquisition, meaning, and transfer) addressed in the learning plan? Yes, learning plan leads to meaningful understanding of material and the transfer of knowledge to different content areas. • Does the learning plan reflect principles of learning and best practices? Yes, learning plan includes multiple modes of instruction and learning. • Is there tight alignment with Stages 1 and 2? Yes, lessons and activities are designed to encapsulate the learning objectives and understandings laid out in Stage 1. The lessons and activities will lead to successful completion of the assessments described in stage 2. • Is the plan likely to be engaging and effective for all students? I believe it will be engaging for all due to the various activities and labs that we will be completing, as well as the various modes of instruction for the material. 4 CHRIS LEVERINGTON APPENDIX A Responses to Analysis Questions How does this unit relate to my internship? My internship was in the field of aerospace engineering, which doesn’t directly relate to my classroom teaching in many ways. Orbital Sciences Corporation builds rockets that launch satellites into space; therefore there aren’t many areas that correlate between the industry and my chemistry classroom. One area that I did find applicable was chemical reactions. There are many chemical reactions that must take place in order to provide the rocket with the thrust required to enter into orbit, as well as to separate the stages of the rocket. The chemical reactions that take place in the rocket are far too complex for a high school introductory chemistry student, but the general idea of chemical reactions does apply in this setting; therefore I chose to focus my unit on chemical reactions. This summer I also took a biochemistry course, a topic of chemistry that is very commonly overlooked in high schools even in the biology courses. In the biochemistry course I kept coming back to how all of the reactions with the body, demonstrate the law of conservation of mass so clearly, that I felt that I could use that to demonstrate that this law is followed in many different settings, so I chose to apply my biochemistry content to this unit as well. The major way that my unit relates to my internship is through the use of workplace skills, often called 21st Century Skills. In my workplace I commonly observed engineers collaborating on projects, going to each other for help, working together to solve problems, self-teaching and communicating their findings to each other through presentations and technical documents. I think that all of these skills are reflected in my unit plan. The students will have to work together to solve problems in lab situations and on the performance task, they will have to collaborate to complete the performance task, and they will need to teach themselves about the Calvin or Citric Acid Cycle. Lastly they will have to communicate their results to their peers to demonstrate what they have learned. All-in-all I feel that with my limited participation in the workings of Orbital Sciences, I have done a decent job of pulling what I could from the experience and putting into this unit plan. What technology resources will be used in this unit? Due to the nature of the content there isn’t much need for advanced technology, nor do I have much technology available. Students will definitely be using computers and the internet in the performance task. They will use the internet to search for information on the Calvin or Citric Acid Cycle and determine how mass is conserved within the processes of photosynthesis or cellular respiration. They will use computers and possibly a video camera and photo editing software to create the presentation to communicate their findings. Conservation of Matter and Chemical Reactions UBD Unit Plan 5 Chris Leverington APPENDIX A Responses to Analysis Questions Item 1: Self-Assessment Rubric Unit Weekly Self-Assessment Rubric This week’s topic: _______________________ Name: ______ Category Doing Great Hangin in There Up a creek Concepts related to this week’s learning Problems/ Calculations I understand all of the concepts discussed I understand most, need help with some (listed below) I understand some of the concepts, but not most of them I feel confident that I can solve the majority of problems learned this week I worked hard on the labs this week and understand their connection to the learning in class I have put a lot of effort into learning the material this week, including work after school. I feel confident that I will be able to contribute to my group regarding the use of this week’s learning on the final assessment I can do most of the problems, but get really stuck on some (listed below) I tried on the labs this week and can see how they relate to the learning in class I have tried to learn material in class this week, but haven’t spent time out of school I feel that with some extra support I will be able to adequately contribute to my group regarding the use of this week’s learning on the final assessment I can’t do any of the problems, I just don’t get it. Labs My Effort Progress toward Final Assessment Questions you have regarding this week’s material? Concepts that you just didn’t understand? What efforts have you made to get extra help? I let my group members do the labs this week We learned something new this week? I don’t think I will be of much help to my group on this portion of the final assessment 6 CHRIS LEVERINGTON Item 2: Law of Conservation of Mass Inquiry Lab Law of Conservation of Matter Inquiry Lab Names: ________________________________ The Task: As a group you will design an experiment that you will use to prove the Law of Conservation of Matter to be true. Once you have written your procedure and gotten it approved, you will carry out the experiment. Materials Available for your use: A Balance Baking Soda A flask A Balloon Vinegar A Graduated Cylinder A weighing boat A thermometer A 250 ml beaker Safety Concerns—and other rules When you are carrying out your experiment you must wear goggles. You are not allowed to mix large amounts of baking soda and vinegar together—Use small amounts please—No more than 12g of baking soda per trial Procedure—Refer to the “Keys to a good procedure” on the board 1. _________________________________________________________ 2. _________________________________________________________ 3. _________________________________________________________ 4. _________________________________________________________ 5. _________________________________________________________ 6. _________________________________________________________ 7. _________________________________________________________ 8. _________________________________________________________ 9. _________________________________________________________ 10. _________________________________________________________ 11. _________________________________________________________ 12. _________________________________________________________ 13. _________________________________________________________ Conservation of Matter and Chemical Reactions UBD Unit Plan 7 Chris Leverington 14. _________________________________________________________ 15. _________________________________________________________ Data Collection—Draw a Data Chart here to use for data collection according to your procedure. Analysis Questions—The questions will be on the smart board, answer them here. You do not need to write the questions down. 1. 2. 3. 4. 5. 8 CHRIS LEVERINGTON Item 3: Precipitate Lab In this lab you will be practicing writing and balancing equations. You will then carry out the reactions of the equations that you wrote. The items letters in parenthesis behind the name of the compound tell you the state of the compound in the reaction. (ppt) means precipitate, flecks of solid formed. (aq) means aqueous, or in solution, no solid formed. (s) means solid, (l) means liquid, (g) means gas. Step 1: Determine the correct chemical equation for each reaction below. Step 2: Check your equation, at the board to see if it is correct. If it is not correct, fix it on your own. Step 3: Once you are sure you have the correct equation, balance the equations, using an atom inventory on a separate sheet of paper. Step 4: Once all equations are correctly written and balanced, you may move on to step 5 on the board. 1. Nickel(ll) chloride (aq) + sodium sulfide (aq) nickel(ll) sulfide (ppt) + sodium chloride (aq) 2. Barium nitrate (aq) +copper(ll) sulfate(aq) barium sulfate (ppt) + copper(ll) nitrate (aq) 3. HCl + calcium carbonate (s) Water (l) + calcium chloride (aq) + carbon dioxide (g) 4. Potassium Chromate (aq) + silver(I) nitrate (aq) -> silver(I) chromate (ppt) +potassium nitrate (aq) 5. Silver (I) nitrate (aq) + nickel(ll) chloride (aq) -> silver chloride (ppt) + nickel (II) nitrate (aq) 6. Cobalt (ll) Nitrate(aq) and sodium hydroxide (aq) cobalt(ll) hydroxide(ppt) + sodium nitrate(aq) 7. Potassium iodide (aq) and lead (ll) nitrate (aq) lead (II) iodide (ppt) + potassium nitrate (aq) Conservation of Matter and Chemical Reactions UBD Unit Plan 9 Chris Leverington Observations Reaction 1 Color of precipitate:________ Reaction 2 Color of precipitate:________ Reaction 3 Color of precipitate:________ Reaction 4 Color of precipitate:________ Reaction 5 Color of precipitate:________ Reaction 6 Color of precipitate:________ Reaction 7 Color of precipitate:________ Questions 1. List the signs of a chemical reaction that we discussed in class that you saw in this lab. Provide an example of which reaction each one occurred in. (Ex: In reaction 1 we saw bubbling, which means a gas was formed.) 2. Choose two of the balanced equations above and write the correct sentence equation for that reaction. (ex: 2 molecules of hydrogen reacted with 2 molecules of oxygen to produce 2 molecules of water.) 1 0 CHRIS LEVERINGTON Item 4: Types of Reactions Lab Types of Reactions Lab *** Do not write on this lab handout*** Objective: The purpose of this lab is to give us some insight into each of the types of reactions that we discussed in class this week. In this lab you will be carryout each type of chemical reaction, make observations regarding the chemical reactions and predict the final products of each reaction. If time permits, I will be doing some larger scale demonstrations of a few of the reactions shown below. Materials Magnesium Ribbon 3 small test tube Hydrochloric Acid Copper(II) Sulfate Solution Hydrogen Peroxide Aluminum Foil Magnesium Pieces 1 small grad. cylinder Large Test Tube Potassium Carbonate Solution dish soap Copper (II) Chloride 4 Wood Splints Watch Glass 3 small beakers 1 Flask Isopropyl Alcohol Graduated Cylinder Safety All hair must be pulled back Safety goggles must be worn at all times Report any spills or accidents to the teacher immediately Do NOT look at the burning magnesium directly The Bunsen burners burn at a very high temperature, do not put your hands in the flame or touch anything that has been in the flame. Do NOT attempt these things at home or without proper supervision For Safety concerns we will carry out each portion of the lab together. Do not work ahead…wait until I instruct you to do the reaction before doing anything. Procedure Reaction 1 In this reaction will be burning magnesium in the presence of oxygen. The reactants of this reaction are Mg + O2 1. Obtain a piece of magnesium metal from the teacher. 2. Ask the teacher to come light your Bunsen burner. 3. Take a hold of the piece of magnesium metal in the tongs provided. 4. Carefully place the piece of magnesium into the flame. Do not look directly at the magnesium as it burns. You can look at someone else’s who is not near you. 5. When the magnesium is finished burning place the remains on a watch glass. 6. Light a wood split on fire in the burner, let it burn down a little ways and blow it out. Place the burnt end near the ashes and record any results in the data chart. 7. Turn off gas supply to the Bunsen burner. Discard ash into trash, wash and dry glass. Conservation of Matter and Chemical Reactions UBD Unit Plan 11 Chris Leverington Reaction 2 In this reaction we will be mixing Magnesium Metal with Hydrochloric Acid. HCl is a corrosive chemical. If it comes in contact with your skin, wash hands immediately and report to teacher. The reactants in this reaction are as follows. Mg + HCl 1. 2. 3. 4. 5. 6. 7. 8. 9. Measure 10ml of Hydrochloric Acid (HCl) and add it to your first small test tube. Take a hold of the 2nd test tube in a test tube holder. Ask the teacher for a piece of magnesium metal. Drop the piece of magnesium into the HCl solution. Immediately place the 2nd test tube upside down over the top of the 1st test tube. Create an airtight seal between the two. Refer to drawing on board. When the bubbling ceases or slows down, ask the teacher to light your Bunsen burner. Light a wood splint on fire, bring it near the mouths of the two test tubes. Turn off gas supply. Pull the test tubes apart slightly and put the lit splint between the two. Record observations in the data table. Reaction 3 In this reaction we will be decomposing Hydrogen Peroxide. In this reaction Potassium Iodide will act as a catalyst. A catalyst is something that speeds up a chemical reaction. Hydrogen Peroxide will decompose slowly over time, by adding the KI, it speeds this process up considerably. The equation is as follows: H2O2 1. 2. 3. 4. 5. Place your large test tube inside your flask. Fill your large test tube approximately ½ full of H2O2. Caution: Soap Place a drop or two of dish soap into the large test tube. bubbles will stain Complete the rest of this lab in or near the sink. skin for a few days if Pour 20 ml of KI into the test tube. touched!! Record your observations on the data chart. Reaction 4 In this reaction we will be mixing two solutions together. We will be copper (II) chloride with Potassium Iodide. The equation is as follows: CuCl2 + KI 1. 2. 3. 4. 5. Place your 3rd test tube in the test tube rack. Put 20 drops KI solution into the test tube. Put 10 drops of the CuCl2 solution into the test tube. Record any observations on the data chart. Dispose of chemicals in the waste bucket in the front of the room. 1 2 CHRIS LEVERINGTON Reaction 5 In this reaction will be burning isopropyl alcohol in the presence of oxygen. It is imperative that all directions are followed on this section 100% correctly. The equation for this reaction is: C3H8O + O2 1. 2. 3. 4. 5. Measure out 5ml of isopropyl alcohol in a small beaker. Pour the alcohol onto your watch glass. Raise your hand and ask the teacher to light your alcohol on fire. Record observations on your data sheet Place a wooden splint into the fire until you see it light, be careful not to burn your fingers. Once it is lit, slowly pull it out of the flame. 6. Hold the splint slightly above the top of the flame. 7. Record any observations you see happening to the fire on the splint in the data chart. Reaction 6 In this reaction we will be mixing silver(I) Nitrate with copper to make Ag. The equation for this reaction is: CuCl2 + Al 1. 2. 3. 4. 5. Measure out 30ml of Copper (II) Chloride into the small flask. Place the piece of aluminum foil into the copper (II) chloride solution. Swirl or Stir the contents of the flask to help the reaction start. Record your observations on your data sheet. Dump contents of flask into waste bucket in front of room!








