Ionic vs Covalent Compounds Lab
advertisement

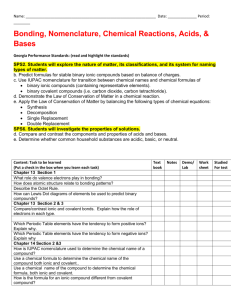
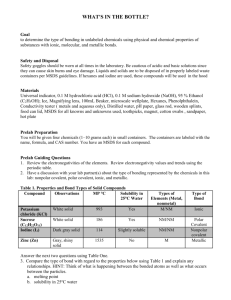
Physical Science Ch 6 Lab Name____________________ Date____________Per______ Ionic vs Covalent Compounds Lab Introduction: Compounds can be classified by the types of bonds that hold their atoms together. Ions are held together by ionic bonds and atoms are held together by covalent bonds. You cannot tell whether a compound contains ionic or covalent bonds just by looking at a sample of it because both types of compounds can look similar. However, simple tests can be done to classify the samples as ionic or covalent because each type of bond has a set of characteristic properties. One property is solubility. Chemists use the term “like dissolves like” when describing whether a substance will be able to dissolve in another substance. Specifically, polar (charged) solvents will only dissolve polar substances and nonpolar (no charge) solvents will only dissolve nonpolar substances. Water is a polar solvent used in part 2 of this lab. Oil is a nonpolar solvent used in part 2. Pre-lab Questions: 1. List 3 properties of ionic compounds. 2. List 3 properties of covalent compounds. (by contrast from question 1) Procedures: 1. Part 1 Melting Point a. Turn on your hotplate and set to medium heat (5). b. Obtain small squares of aluminum foil. c. Place a small scoop of the first substance in the center of the aluminum and heat on the hotplate. d. Remove before the substances start to burn. e. Record in your data table whether the substance has a low melting point (melts quickly) or a high melting point (doesn’t melt at all). f. Repeat the procedure for all remaining substances. g. Throw away all materials when complete. 2. Part 2 Solubility a. Put approximately 5 mL of water in one test tube. b. Put approximately 5 mL of oil in a second test tube. c. Place a small scoop of the first substance in each test tube. d. Cap each test tube and shake. e. Record your data as either dissolving or not dissolving in the water or oil. f. Dispose of oil and water in designated containers. g. Repeat procedure for all remaining substances. 3. Part 3 Teacher Demo Conductivity Data: Substance NaCl Sucrose (sugar) Starch Crisco NaI Wax NaHCO3 LiCl Melting Point (choose High or Low) Solubility (choose oil, water, both or neither) Conductivity (choose positive or negative) Classification (Choose Ionic or Covalent) Analyze and Conclude: 1. Classifying: Complete your data table above by classifying each of the substances you tested as ionic or covalent compounds based on your observations. 2. How did the melting points of the ionic compounds and the covalent compounds compare? 3. How did conductivity of the ionic and covalent compounds compare? 4. What happened to the bonds between the molecules when a substance melted? 5. Determine, based on your lab results, whether each of the substances listed would be ionic or covalent. a. Silica m.p. 1600°C b. Magnesium chloride m.p. 1412°C c. Oleic Acid m.p. 14°C, d. Lye m.p. 318°C e. Petroleum Jelly m.p. 37°C f. Glucose m.p. 146°C g. Epson Salt m.p. 1124°C h. Gasoline m.p. -57°C