FLAME TEST LAB
advertisement

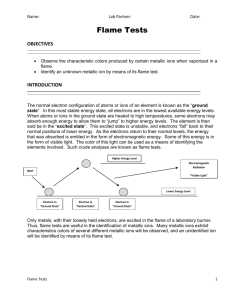
Flame Test Lab 12/09/13 OHS Chemistry Background: The normal electron configuration of atoms or ions of an element is known as the "ground state". In this most stable energy state, all electrons are in the lowest energy levels available. When atoms or ions in the ground state are heated to high temperatures, some electrons may absorb a specific amount of energy (a quantum) to allow them to "jump" to higher energy levels. The element is then said to be the "excited state". This excited configuration is unstable, and the electrons "fall" back to their normal positions of lower energy. As the electrons return to their normal levels, the energy that was absorbed is emitted in the form of electromagnetic energy. Some of the energy may be in the form of visible light. The color of this light can be used as means of identifying the elements involved. Such crude analyses are known as flame tests. Only metals, with their loosely held electrons, are excited in the flames of a laboratory burner. Thus, flame tests are useful in the identification of metallic ions. Many metallic ions exhibit characteristic colors when vaporized in the burner flame. PURPOSE: Observe the characteristic colors produced by several metallic ions when vaporized in a flame. Identify an unknown metallic ion by means of its flame test. EQUIPMENT and MATERIALS: Laboratory burner Safety goggles Q-tips Diffraction grating Cobalt Blue Glass Aprons Beaker Colored pencils Cation Solutions (each 0.5 molar solution contains a metallic ion with a NO3 anion): Na+, K+, Li+, Ca2+, Sr2+, Ba2+, Cu2+, B+3 Unknown Solutions PROCEDURE: 1. Put one end of a Q-tip into a solution bottle, being careful not to touch the other end. 2. Insert the moistened Q-tip end into the hottest part of the flame, allowing the chemical solution to burn, showing its characteristic color. Avoid leaving the Q-tip in the flame too long as the Q-tip itself will begin to burn and its burn color may be confused with the ion color. 3. Observe and record any new colors noted in the flame. 4. Perform a second test using the unburned end of your Q-tip. 5. Discard Q-tips in the large beaker after both ends have been used or if any contamination occurs. Do not put the Q-tips in the trash. 6. Repeat the first 5 steps using the other known ion samples until a definitive flame color can be observed for each solution. Record data. 7. Using the diffraction grating view the flame’s spectrum. Using colored pencils record the bright line spectrum in your data table 8. Repeat the same steps for the three unknown solutions. Record data. Match your results for each unknown to one of the seven known chemicals previously tested. Record. IMPORTANT NOTES: Be VERY careful not to contaminate bottles of metal nitrate solutions. Cobalt blue glass may be used with the potassium ion solution to filter out interfering wavelengths of light. View the flame through the glass. It is helpful to lower the light level in the room to achieve better contrast. OBSERVATIONS AND DATA: Metallic Ion: Color of Flame Spectrum Ca2+ Li+ B3+ K+ Ba2+ Na+ Cu2+ Sr2+ Identity of Unknown Unknown 1 Unknown 2 Unknown 3 QUESTIONS: 1. Which pairs of ions produce similar colors in the flame tests? What can be done to avoid confusion in your identification of these ions? 2. Define these terms: (a) quantum (b) ground state (c) excited state 3. What is a spectroscope? What is observed if the flame tests are viewed through a spectroscope? 4. This is a qualitative lab. How could it be modified to quantify the data? i.e. What kind of measurements could be made to determine the amount and/or type of colored light emitted?