February 7-11 - Types of Reactions
advertisement

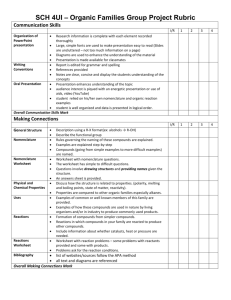
Teacher: Ms. Erin Hopkins Chemistry Unit 2: Chemical Reactions January 31st – February 4th Pennsylvania Standards: Standard - 3.2.C.A4: - Predict how combinations of substances can result in physical and/or chemical changes. - Interpret and apply the laws of conservation of mass, constant composition (definite proportions), and multiple proportions. - Balance chemical equations by applying the laws of conservation of mass. - Classify chemical reactions as synthesis (combination), decomposition, single displacement (replacement), double displacement, and combustion. - Use stoichiometry to predict quantitative relationships in a chemical reaction. Pennsylvania Anchors: CHEM.A.1.1: Identify and describe how observable and measurable properties can be used to classify and describe matter and energy. CHEM.A.1.2: Compare the properties of mixtures. CHEM.B.1.1: Explain how the mole is a fundamental unit of chemistry. CHEM.B.1.2: Apply the mole concept to the composition of matter. CHEM.B.1.3: Explain how atoms form chemical bonds. CHEM.B.1.4: Explain how models can be used to represent bonding. CHEM.B.2.1: Predict what happens during a chemical reaction. CHEM.B.2.2: Explain how the kinetic molecular theory relates to the behavior of gases. CHEM.A.1.1.5: Apply a systematic set of rules (IUPAC) for naming compounds and writing chemical formulas (e.g. binary covalent, binary ionic, ionic compounds containing polyatomic ions). 1 Wednesday Student Objectives Essential Questions Teaching & Enrichment Activities/materials needed The students will be able to: 1. Identify and name binary ionic compounds. 2. Identify and name tertiary ionic compounds. 3. Identify and name molecular compounds. 4. Identify and name acids. 5. Explain the rules of binomial nomenclature for chemical compounds. BIG IDEA: Chemical reactions are predictable. TOPIC: Chemical Nomenclature - -----------------------------------------------------------VOCABULARY: ionic compounds, covalent compounds, binary ionic compounds, tertiary ionic compounds, molecular compounds, acids ESSENTIAL QUESTION: What are the rules for naming chemical compounds? TEACHING ACTIVITIES/STRATEGIES: Warm Up: Students will be asked to take out their Chemical Nomenclature Video Presentation handout. Students will work in their video presentation groups and will be given 5 minutes to check their answers and make sure they have all of the flash cards and title slides they need for their video. Video Taping: Students will receive a rubric that will describe the advisors expectations for their video presentation. Students will then read the rubric as a class, and the advisor will answer any questions that students may have. Students will be given the rest of the class period to film their video presentations. Each group will be required to divide the tutorial into sections to be presented by each group member. At the end of class, students will return all supplies and hand in their Chemical Nomenclature Video Presentation handout. HOMEWORK: Students will be required to finish their video presentations during project time, after school, or at home if they do not finish them in class. Students will have one week to finish these videos outside of class, but will not be given any more class time. ASSESSMENT: Students will be assessed on their understanding of the chemical naming system using the rubric that was created for the video presentation. MATERIALS: Chemical Nomenclature Video Presentation handouts Flash Cards Markers Colored Pencils Chemistry Textbooks Computers with internet access 2 Friday The students will be able to: 1. Distinguish among the five major types of chemical reactions. 2. Classify a reaction as belonging to one of five major types. 3. Observe physical changes that take place during chemical reactions. 4. Compare changes that take place during different types of chemical reactions. BIG IDEA: Chemical reactions are predictable. ESSENTIAL QUESTION: 1. How do stoichiometric ratios relate reactants to products in a chemical reaction? 2. What factors identify the types of chemical reactions? 3. According to the collision theory, what factors affect the rate of a chemical reaction? Video cameras TOPIC: Types of Reactions VOCABULARY: synthesis, decomposition, single displacement, double displacement, combustion TEACHING ACTIVITIES/STRATEGIES: ChemLab: Types of Reactions Pass out the lab handout and read the background information and essential question aloud to the class. Tell students they will be working in groups to complete four reactions at four different stations. Explain to students that they will be given 10-15 minutes at each station. Ask a student to read through the procedure for the first reaction on the lab handout and demonstrate the tasks as the student reads the directions. Demonstrate each station as a student reads the procedure aloud. Go over proper use of the Bunsen burner and the set-up for collecting the gas given off in the single-replacement reaction. After discussing the directions for the lab, divide students into groups of three and pass out goggles and aprons (students should be placed in heterogeneous groups). Tell students to put the goggles and aprons on and to keep them on until all chemicals are cleaned up and put away at the end of class. As students work in groups at the different stations, walk around the classroom to monitor student progress. Make sure that students are wearing goggles at all times and that they are following the directions on the lab handout. Closely monitor students performing the single-replacement reaction and the synthesis reaction. This lab may take two class periods – collect the lab handouts at the end of the first class period to ensure that students will have the lab handout for the next class period. HOMEWORK: Students will need to answer the Analysis Questions on the lab handout once the lab is complete. 3 ASSESSMENT: Students will be assessed on their answers to the questions on the lab handout. MATERIALS: ChemLab: Types of Reactions handout Goggles and aprons Assorted beakers Test tubes Test tube clamps Bunsen burner Tongs Magnesium Ribbon (Mg) Calcium carbonate (CaCO3) Sodium carbonate (Na2CO3) Calcium chloride (CaCl2) Calcium hydroxide solution (Ca(OH)2 – lime water) Powdered sulfur (S) Hydrochloric acid (HCl) Mossy Zinc (Zn) Wooden splint Graduated cylinders 4 MATERIALS: 5