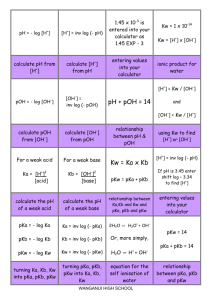
TITRATION CURVES (2)
advertisement
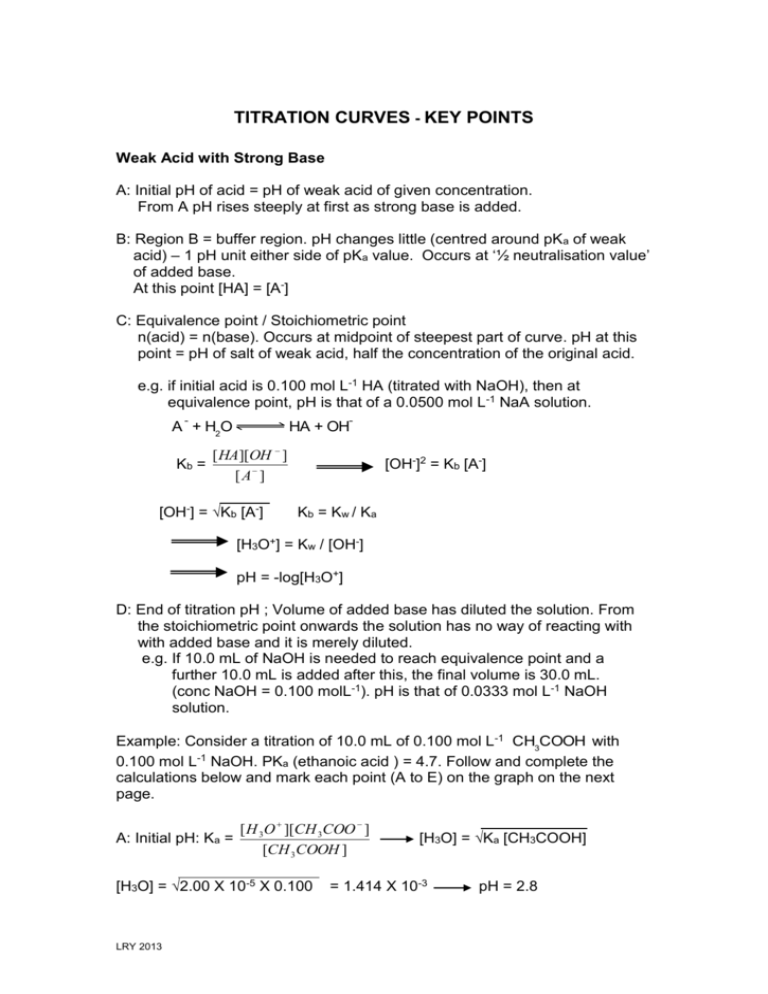
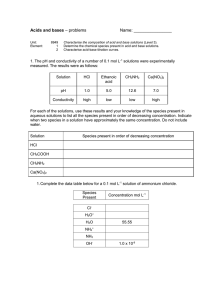
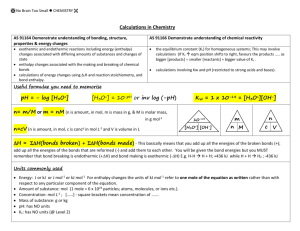
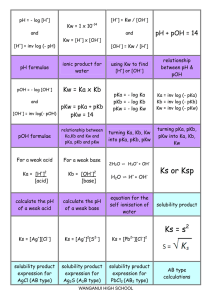
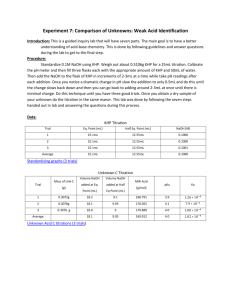
TITRATION CURVES - KEY POINTS Weak Acid with Strong Base A: Initial pH of acid = pH of weak acid of given concentration. From A pH rises steeply at first as strong base is added. B: Region B = buffer region. pH changes little (centred around pKa of weak acid) – 1 pH unit either side of pKa value. Occurs at ‘½ neutralisation value’ of added base. At this point [HA] = [A-] C: Equivalence point / Stoichiometric point n(acid) = n(base). Occurs at midpoint of steepest part of curve. pH at this point = pH of salt of weak acid, half the concentration of the original acid. e.g. if initial acid is 0.100 mol L-1 HA (titrated with NaOH), then at equivalence point, pH is that of a 0.0500 mol L-1 NaA solution. -- -- A + H2O Kb = HA + OH [ HA][OH ] [ A ] [OH-] = Kb [A-] [OH-]2 = Kb [A-] K b = K w / Ka [H3O+] = Kw / [OH-] pH = -log[H3O+] D: End of titration pH ; Volume of added base has diluted the solution. From the stoichiometric point onwards the solution has no way of reacting with with added base and it is merely diluted. e.g. If 10.0 mL of NaOH is needed to reach equivalence point and a further 10.0 mL is added after this, the final volume is 30.0 mL. (conc NaOH = 0.100 molL-1). pH is that of 0.0333 mol L-1 NaOH solution. Example: Consider a titration of 10.0 mL of 0.100 mol L-1 CH3COOH with 0.100 mol L-1 NaOH. PKa (ethanoic acid ) = 4.7. Follow and complete the calculations below and mark each point (A to E) on the graph on the next page. A: Initial pH: Ka = [ H 3O ][CH 3COO ] [CH 3 COOH ] [H3O] = 2.00 X 10-5 X 0.100 LRY 2013 [H3O] = Ka [CH3COOH] = 1.414 X 10-3 pH = 2.8 Titration Curve C: At equivalence point – PH = that of 0.0500 mol L-1 solution of CH3COONa. [OH-] = Kb[CH3COO-] = = [H3O+] = pH = pH B: At ½ way to equivalence point pH = pKa = 4.7 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 0 D: At 20 mL of added NaOH, pH is that of 0.0333 mol L-1 NaOH. [OH-] = 0.0333 mol L-1 [H3O+] = pH = 2 4 6 8 10 12 14 16 18 20 vol NaOH (mL) E: At 15 mL added base – the unreacted base = 15 – 10 mL = 5 mL Therefore n(unreacted NaOH) = 0.100 x 5 x 10-3 mol = Total volume = 25 mL. Therefore [NaOH] = = mol L-1 [OH-] = [H3O+] = pH = Choice of indicator: The best indicator for this titration is one which changes colour in the steepest part of the curve. The pH at the end point is 8.7 and since an indicator changes colour over a range of 2 pH units near its pKa value, we need an indicator whose pKa value lies in that region of the curve. What would be suitable? LRY 2013