Experiment Determination of ions concentration in the solution.
advertisement
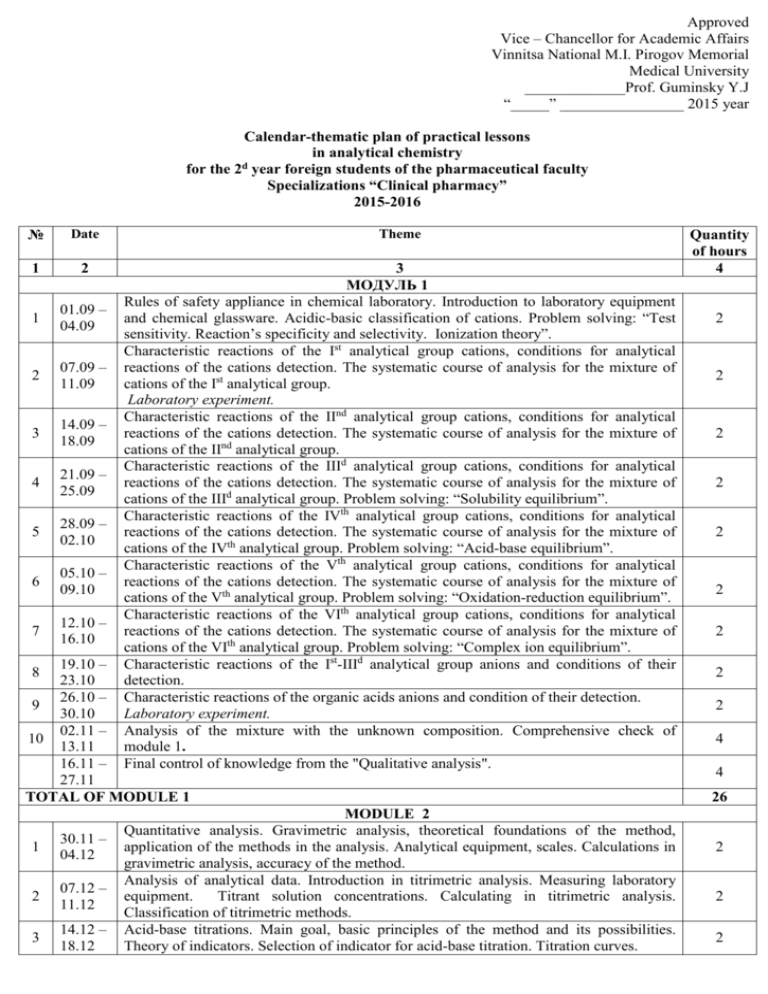
Approved Vice – Chancellor for Academic Affairs Vinnitsa National M.I. Pirogov Memorial Medical University _____________Prof. Guminsky Y.J “_____” ________________ 2015 year Calendar-thematic plan of practical lessons in analytical chemistry for the 2d year foreign students of the pharmaceutical faculty Specializations “Clinical pharmacy” 2015-2016 № Date Theme 1 2 1 01.09 – 04.09 2 07.09 – 11.09 3 14.09 – 18.09 4 21.09 – 25.09 5 28.09 – 02.10 6 05.10 – 09.10 7 12.10 – 16.10 3 МОДУЛЬ 1 Rules of safety appliance in chemical laboratory. Introduction to laboratory equipment and chemical glassware. Acidic-basic classification of cations. Problem solving: “Test sensitivity. Reaction’s specificity and selectivity. Ionization theory”. Characteristic reactions of the Ist analytical group cations, conditions for analytical reactions of the cations detection. The systematic course of analysis for the mixture of cations of the Ist analytical group. Laboratory experiment. Characteristic reactions of the IInd analytical group cations, conditions for analytical reactions of the cations detection. The systematic course of analysis for the mixture of cations of the IInd analytical group. Characteristic reactions of the IIId analytical group cations, conditions for analytical reactions of the cations detection. The systematic course of analysis for the mixture of cations of the IIId analytical group. Problem solving: “Solubility equilibrium”. Characteristic reactions of the IVth analytical group cations, conditions for analytical reactions of the cations detection. The systematic course of analysis for the mixture of cations of the IVth analytical group. Problem solving: “Acid-base equilibrium”. Characteristic reactions of the Vth analytical group cations, conditions for analytical reactions of the cations detection. The systematic course of analysis for the mixture of cations of the Vth analytical group. Problem solving: “Oxidation-reduction equilibrium”. Characteristic reactions of the VIth analytical group cations, conditions for analytical reactions of the cations detection. The systematic course of analysis for the mixture of cations of the VIth analytical group. Problem solving: “Complex ion equilibrium”. Characteristic reactions of the Ist-IIId analytical group anions and conditions of their detection. Characteristic reactions of the organic acids anions and condition of their detection. Laboratory experiment. Analysis of the mixture with the unknown composition. Comprehensive check of module 1. Final control of knowledge from the "Qualitative analysis". 19.10 – 23.10 26.10 – 9 30.10 02.11 – 10 13.11 16.11 – 27.11 TOTAL OF MODULE 1 8 1 30.11 – 04.12 2 07.12 – 11.12 3 14.12 – 18.12 MODULE 2 Quantitative analysis. Gravimetric analysis, theoretical foundations of the method, application of the methods in the analysis. Analytical equipment, scales. Calculations in gravimetric analysis, accuracy of the method. Analysis of analytical data. Introduction in titrimetric analysis. Measuring laboratory equipment. Titrant solution concentrations. Calculating in titrimetric analysis. Classification of titrimetric methods. Acid-base titrations. Main goal, basic principles of the method and its possibilities. Theory of indicators. Selection of indicator for acid-base titration. Titration curves. Quantity of hours 4 2 2 2 2 2 2 2 2 2 4 4 26 2 2 2 1 2 4 21.12 – 25.12 5 28.12 – 01.01 04.01 – 08.01 3 Titration of strong acids by strong bases vice versa. Titration of weak acids by strong bases, titration weak bases by strong acids. Laboratory experiment. Preparation of hydrochloric acid and sodium hydroxide solutions and their standardization. Quantitative determination of acetic acid. Seminar "Gravimetry and acid-base titration". Comprehensive check of module 2. Redox titration. Classification of methods. Indicators that are used in redox titrations.. Permanganatometry. Iodometry and iodimetry 11.01 – 7 Experiments. Preparation and standardization of sodium thiosulfate solution. 15.01 Quantitative determination of ascorbic acid TOTAL OF MODULE 2 TOTAL 6 Head of the Pharmaceutical Chemistry Department 4 2 2 2 2 14 40 PhD Assistant Professor Yuschenko T.I. Approved Vice – Chancellor for Academic Affairs Vinnitsa National M.I. Pirogov Memorial Medical University _____________Prof. Guminsky Y.J “_____” ________________ 2015 year Calendar-thematic plan of practical lessons in analytical chemistry for the 2d year foreign students of the pharmaceutical faculty Specializations “Clinical pharmacy” 2015-2016 № п/п 1 Date 2 Theme 3 MODULE 3 Optical methods, classification. Refractometry, using in qualitative and quantitative 1 analysis. Photometry and spectrofotometry. Conditions in photometry, the general methods of 2 determination concentrations of the solution. Experiment Photometric determination of iron in the solution. 3 Seminar "Optical methods". Comprehensive check of module 4. Electrochemical methods of analysis. Classification of electrochemical methods, the 4 description of direct and indirect electrochemical methods. Conductometry, classification. Potentiometric methods of analysis, classification. 5 Experiment Determination of ions concentration in the solution. Chromatographic methods in analysis, classification. Ion-exchange chromatography. Thin-layer and paper chromatography. Utilization in analysis. 6 Experiments Determination and separation of ions using the precipitation chromatography on the paper. Identification of the substances using the thin – layer chromatography. 7 Gas chromatography. Utilization in analysis. Seminar «Electrochemical methods and Chromatographic methods of analysis». 8 Comprehensive check of module 5. Final control of knowledge «Instrumental methods of analysis». TOTAL OF MODULE 3 TOTAL Quantity of hours 4 2 4 2 2 2 2 2 2 4 22 76







