a=Edge of the unit cell
advertisement
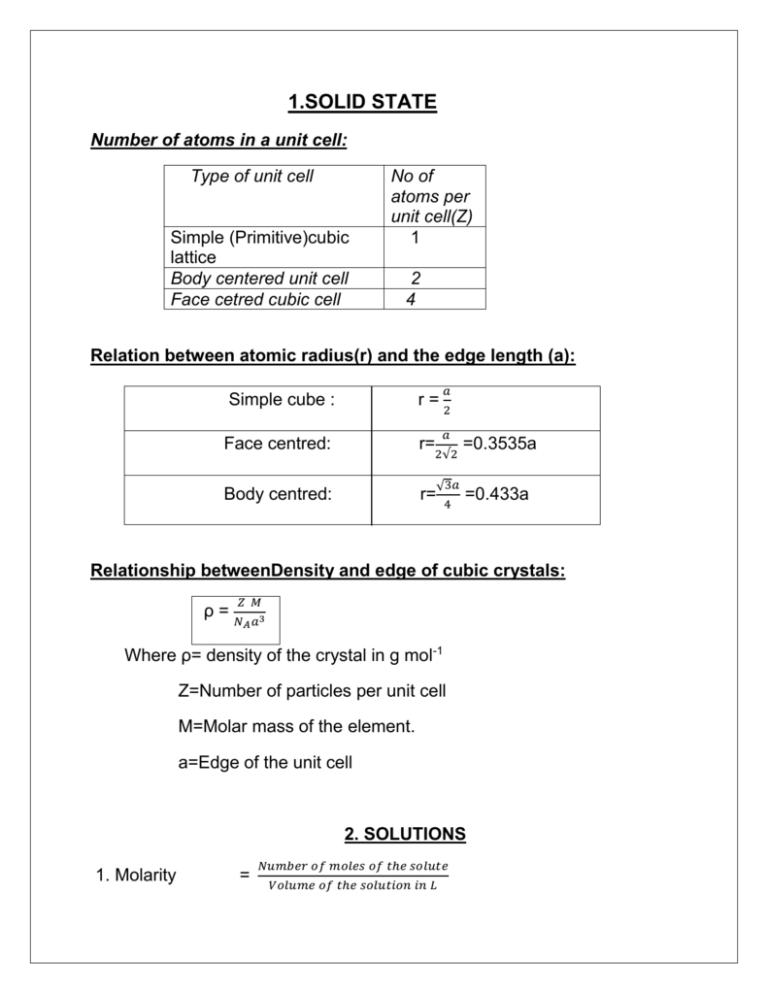
1.SOLID STATE Number of atoms in a unit cell: Type of unit cell Simple (Primitive)cubic lattice Body centered unit cell Face cetred cubic cell No of atoms per unit cell(Z) 1 2 4 Relation between atomic radius(r) and the edge length (a): Simple cube : r= Face centred: r= Body centred: r= 𝑎 2 𝑎 2√2 √3𝑎 4 =0.3535a =0.433a Relationship betweenDensity and edge of cubic crystals: ρ= 𝑍 𝑀 𝑁𝐴 𝑎3 Where ρ= density of the crystal in g mol-1 Z=Number of particles per unit cell M=Molar mass of the element. a=Edge of the unit cell 2. SOLUTIONS 1. Molarity = 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑡ℎ𝑒 𝑠𝑜𝑙𝑢𝑡𝑒 𝑉𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑡ℎ𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑖𝑛 𝐿 2. Mole fraction = 3. Molality 𝑛1 𝑛1 +𝑛2 𝑁𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 = 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑖𝑛 𝐾𝑔 Henry’s law: The mass of a gas dissolved in a given volume of of the liquid at constant temperature is directly proportional to the pressure of the gas present in equilibrium with the liquid. m = kH p The widely used form of Henry’s Law is “The partial pressure of a gas in vapour phase is directly proportional to the mole fraction of the gas in the solution.” 𝒫𝒜= 𝒦ℋ 𝒳𝒜 Raoult’s law: “The vapour pressure of a solution containing non-volatile solute is directly proportional to the mole fraction of the solvent.” 𝒫 A=𝒫 ◦A𝒳 A Raoult’s law as a special case of Henry”s law: Comparing the two laws, the following conclusion can be drawn: Pressure of the volatile component of a solution is directly proportional to its mole fraction. The two equations become identical when KH becomes equal to 𝒫 ◦A . Colligative properties: Those properties which depend only on the number of particles of the solute not on the nature of the solute particles. Relative lowering of vapour pressure: 𝜟𝒑 = 𝒑𝟎 𝑨 𝒑𝟎 𝑨−𝒑𝑨 𝒑𝟎 𝑨 = 𝓧B = 𝑾 𝑩 ⁄𝑴 𝑩 𝑾𝑨 ⁄𝑴𝑨+ 𝑾𝑩 ⁄𝑴𝑩 Δp= Depression in vapour pressure 𝑝0 𝐴=Vapour pressure of pure solvent 𝒫𝒜 = 𝑉𝑎𝑝𝑜𝑢𝑟 𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝒳ℬ = 𝑚𝑜𝑙𝑒 𝑓𝑟𝑎𝑐𝑡𝑖𝑜𝑛 𝑜𝑓 𝑐𝑜𝑚𝑝𝑜𝑛𝑒𝑛𝑡 𝐵(𝑆𝑜𝑙𝑢𝑡𝑒) 𝒲A=Mass of component A(solvent) Elevation in boiling point: ΔTb=𝒦 b m= 𝒦 b 𝑊𝐵 ×1000 𝑀𝐵 𝑊𝐴 ΔTb= Elevation in boiling point 𝒦 b = molal elvation constant in K Kg mol-1 m = molality( mol/g) WB= Mass of solute(g) MB= Molar mass of solute WA =Mass of solvent in g Kb = 𝑀𝐴 𝑅𝑇𝑏2 𝛥𝑣𝑎𝑝 𝐻×1000 Kb = Molal elevation constant (K Kg mol-1) MA=molar mas of solvent R= Universal gas constant (8.314 J K-1mol-1; 8.314*10-2 L bar K-1mol-1; 8.21*10-2 L atm K-1mol-1) 𝛥𝑣𝑎𝑝 𝐻=Enthalpy of vaporization of solvent 𝑇𝑏 = 𝐵𝑜𝑖𝑙𝑖𝑛𝑔 𝑝𝑜𝑖𝑛𝑡 𝑜𝑓 𝑝𝑢𝑟𝑒 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 Depression in freezing point: ΔT f= K f m ΔTf= 𝐾𝑓 ×𝑊𝐵 ×1000 𝑀𝐵 𝑊𝐴 Kf= Molal freezing constant Determination of molar mass from osmotic pressure: 𝜋= 𝑊𝐵 ×𝑅𝑇 𝑉𝑀𝐵 (𝑂𝑅) 𝜋 = 𝐶𝑅𝑇 Π = Osmotic pressure C= Molarity V= Volume of solution in L Units of pressure: 1 bar = 10 5 pascal 1atm = 1.01*10 5 Pa =760 torr = 1.01 bar = 760 mm Hg 1 mmHg= 1 torr 1 torr= 1.33*10 -3 bar VAN’T HOFF’S FACTOR: 𝑂𝑏𝑠𝑒𝑟𝑣𝑒𝑑 𝑣𝑎𝑙𝑢𝑒 𝑜𝑓 𝑐𝑜𝑙𝑙𝑖𝑔𝑎𝑡𝑖𝑣𝑒 𝑝𝑟𝑜𝑝𝑒𝑟𝑡𝑖𝑒𝑠 i = 𝑁𝑜𝑟𝑚𝑎𝑙 𝑣𝑎𝑙𝑢𝑒 𝑜𝑓 𝑐𝑜𝑙𝑙𝑖𝑔𝑎𝑡𝑖𝑣𝑒 𝑝𝑟𝑜𝑝𝑒𝑟𝑡𝑖𝑒𝑠 (𝑎𝑠𝑠𝑢𝑚𝑖𝑛𝑔 𝑛𝑜 𝑎𝑠𝑠𝑜𝑠𝑖𝑎𝑡𝑖𝑜𝑛 𝑜𝑟 𝑑𝑖𝑠𝑠𝑜𝑠𝑖𝑎𝑡𝑖𝑜𝑛) 𝑖−1 𝛼= 𝑛−1 𝛼 =the degree of dissociation i= Vant’ Hoff factor i=1 if the solute behaves normally i>1 if solute undergoes dissociation 𝛼= 𝑖−1 𝑛−1 i<1 if the solution undergoes association 𝛼= 1−𝑖 1 𝑛 1− n = no of ions produced on dissociation/no of ions undergoing association. 3.ELECTROCHEMISTRY For the reaction aA+ bB cC +dD Nernst equation to calculate the electrode potential of the cell is E cell= E◦cell - 0.0591 𝑛 log [𝐶]𝑐 [𝐷]𝑑 [𝐴]𝑎 [𝐵]𝑏 STANDARD ELECTRODE POTENTIAL: E0cell= E0cathode - E0anode AT EQUILLIBRIUM, Ecell= 0 ,then E0cell= 𝟎.𝟎𝟓𝟗 𝒏 log Kc GIBB’S FREE ENERGY/MAXIMUM WORK DONE ΔGo= -nF E0cell G= 1 𝑅 𝑎 ρ=R 1 𝜌 G*=R 𝜅 𝜅×1000 𝐶 Λm= 𝜅 Λm=𝐶∗1000 C= mol L-1; 𝛼= 𝛬𝑐𝑚 𝛬°𝑚 G= Conductance, a= area, 𝑙 𝜅= where R= Resistance l=length 𝜅=conductivity; ρ=resistivity G*= cell constant Λm=molar conductivity in S cm2 mol-1 k=conductivity in S cm-1 C= molarity in mol/dm3 ( 1dm3=1000cm3) Λm =molar conductivity in S m2 mol-1 K = conductivity in S m-1 C =molarity in mol/m3 ( 1m3=1000L; 1L=1/1000m3;L-1 = 1000 m-3) 𝛬𝑐𝑚 =molar conducitivty of solutions at any concentration. 𝛬°𝑚 =limiting molar conductivity. 4. CHEMICAL KINETICS Rate= 𝑐ℎ𝑎𝑛𝑔𝑒 𝑖𝑛 𝑡ℎ𝑒 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡 𝑜𝑟 𝑝𝑟𝑜𝑐𝑢𝑐𝑡 𝑡𝑖𝑚𝑒 𝐹𝑜𝑟 𝑡ℎ𝑒 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 𝐴 𝐵 Rate= -d[R] = d[P] dt dt Rate= K [A]α[𝐵]𝛽 Order=𝛼 + 𝛽 Unit for k: Zero order reaction =mol L-1 s-1 First order reaction =sec-1 Second order reaction =L mol-1 sec-1 Differential rate equation: 𝐹𝑜𝑟 𝑡ℎ𝑒 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 𝐴 − 𝑑[𝑅] 𝑑𝑥 𝐵 = 𝑘[𝑅]𝑛 (For n order) Integrated rate equation: 1. Zero order 1 𝑘 = 𝑡 [[𝑅]0− [𝑅]] (Rate of reaction= Rate constant ) 2. First order reaction: k= 2.303 [𝑅]0 𝑙𝑜𝑔 𝑡 [𝑅] 3. First ordeReaction for gaseous substances k= 2.303 𝑝𝑖 𝑙𝑜𝑔 𝑡 (2𝑝𝑖 − 𝑝𝑡 ) where pi =initial pressure, pt = total pressure Half life period: Zero order: 𝑡1/2 = [𝑅]0 2𝑘 First order: 𝑡1 2= 0.693 𝑘 Arrhenius Equation: K= A𝑒 −𝐸𝑎⁄𝑅𝑇 𝑒 −𝐸𝑎⁄𝑅𝑇 = Fraction of molecules that have energy equal to or greater than Ea 𝐄𝐚 Log k= logA - log 𝑘2 𝑘1 = 𝐸𝑎 2.303 𝑅 𝟐.𝟑𝟎𝟑𝐑𝐓 [ 𝑇2− 𝑇1 𝑇2 𝑇1 ]