Standard System of Units and Measurement
advertisement
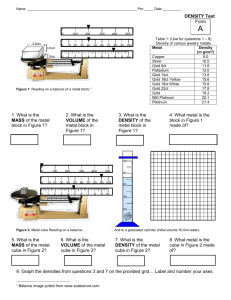
EXPERIMENTS – Ch. 2 Standard System of Units and Measurement Key Terms: Metric system, SI system of units and measures, derived units, dimensions, dimensional analysis, conversion ratios, equalities, arithmetic mean or average, significant figures, rounding, scientific notation, degree of uncertainty, density, specific gravity, Archimedes principle, hydrometer, buoyancy, increments, gradations I Area Determination of Rectangular Index Cards Chemicals: Equipment and Materials: none ruler with in and cm gradations index cards (small, medium, large) Procedure: 1. Measure each side of a rectangular index card (length and width), once in inches and once in centimeters to the nearest hundredth (0.01), if possible 2. Repeat the measurement two more times and tabulate data. 3. Calculate the mean or average for each set of data. 4. Record results using the following table: Observations and Results: Width: Small index card in Medium index card cm in Large index card cm in cm 1 2 3 Mean: Length: Small index card in 1 Medium index card cm in Large index card cm in cm 2 3 Mean: Questions and Problems: 1. What is the conversion ratio of in and cm? Express the ratio in 2. 3. 4. 5. 6. 𝑐𝑚 𝑖𝑛 𝑖𝑛 and 𝑐𝑚. Calculate the area of the rectangular index cards in2 an cm2 by using the mean values. Determine the conversion ratio between in2 and cm2. Verify your measured results by comparison with literature values. What is the degree of error? A photograph is 8x10 in. What is the corresponding size in cm? II Volume Determination of Regular Shaped Objects by Calculation Chemicals: none Equipment and Materials: ruler and caliper plastic cube and metal slug (cylinder) Procedure: 1. Determine the volume of the plastic cube by measuring the length, width and depth (a x a x a or a3) using a metric ruler. Tabulate the results in m, cm and mm values. 2. Determine the volume of a metal slug by measuring the diameter of the circular end with a caliper and the height or length with a metric ruler. 3. Calculate the area of the circular end with the formula A=πr2 (area of a circle) and multiply the result by the determined length or height of the cylinder to give the volume (V=Ah or V= πr2h) 4. Express the volumes in cm3 or mL. Observations and Results: Plastic cube: Side length (a) = ……….m ……….cm ……….mm Volume (a3) = Metal slug: Area of circular end: (A=πr2 , r = d/2) =……….cm2 Volume of cylinder: (V = Ah) = ………..cm3/mL ……….m3 ……….cm3 or mL ……….mm3 Questions and Problems: 1. A liter is defined as 1 dm3. Convert the obtained values for the volume of a cube and metal slug into liters or dm3. 2. What would be the volume of a glass marble, for which the caliper measured a diameter of 12.0 mm? (Volume of a sphere = 4/3 πr3) III Density determination of Regular and Irregular Shaped Objects by the Volume Displacement Method (Archimedes Principle) Chemicals: Equipment and Materials: Tap water Sugar cubes or rock sugar Hexane graduated cylinders (100mL and 250 mL) metal slugs, rubber stoppers, corks Procedure: 1. Mass the solid sample on a digital top-loading balance (scale). 2. Pour a random amount of liquid into the graduated cylinder, submerge the solid object (if less dense than the liquid push down with a pin or thin piece of wire) and record displaced volume (Final volume minus initial volume). Observations and Results: Tabulate the observed results and add the other information required according to the following table: Sample mass (g) dV(mL) D Liquid Vf - Vi (g/mL) used Metal slug Irregular solid Rubber stopper Cork stopper Rock sugar or sugar cube Questions and Problems 1. Why should the object be insoluble in the liquid used for the liquid displacement method? 2. Why does the displacement method work for any kind of liquid? 3. If the object floats in water, what density is best suitable for a liquid that allows the object to become completely submerged? 4. Why is the error by pushing down an object that is less dense then the liquid used with a thin wire negligible when determining the density? 5. A solid object with a mass of 3.55g displaces a volume of 12.0 mL. What is its density? 6. Why is density a material constant? IV Specific Gravity Determination of Solutions using an Hydrometer Chemicals: Sugar solutions of two concentrations: with red dye (higher conc.) with yellow dye (lower conc.) Equipment and Materials: three large graduated cylinders (250-500mL) Hydrometers Procedure: 1. Place the whole amount of the provided sugar solutions each in a tall graduated cylinder. Into the third one put an equivalent amount of demineralized water. 2. Place hydrometers into the each of the three samples and measure the specific gravity (increasing values from top to bottom), provided the hydrometers float in the solutions or in the pure water. Observations and Results: Solution: Water (H2O) Red sugar solution (sucrose) Yellow sugar solution (sucrose) Specific gravity Density (g/mL) Questions and Problems: 1. 2. 3. 4. How is specific gravity defined? Why does specific gravity have no units? Why is the numerical value for specific gravity and density the same for any chemical or object? What is an alloy? How does the specific gravity or density relate to those of the pure metals involved? 5. What is the specific gravity of Gold? How much would a cube with a side-length of 1.5cm weigh?