Chem. Unit 2.4, 11.1 to 11.7 Chapter Checkup key
advertisement

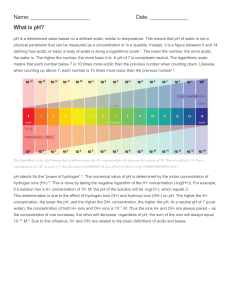
Answers for Units 2.4, 11.1 – 11.6 and Chapter 11 Checkup pg. 164-165 Chem. Unit 2.4 page 23 1-6 1. By simple distillation; see the apparatus at the top of page 22 2. Their purpose is to allow the solvent to condense. The cold water keeps the temperature down, to allow condensation to take place. 3. Because this is a mixture of two liquids, not a solution containing dissolved salts 4. Taking the fractional distillation of a water /ethanol mixture as example: the glass beads in the fractionating column provide a large surface area on which condensation can take place. Once the beads warm up to 78 C, the ethanol passes over into the condenser. But the water vapor condenses on the beads and drips back into the flask. 5. Because there is no green dot at X to match the dot for C 6. You could cut out each colored circle separately, and soak it in a little water. The dye will move into the water. You could decant the liquid (or use a centrifuge) to separate it from the paper, and evaporate the water to obtain the solid dye. (You will be working with very tiny quantities!) Chem. Unit 11.1 page 149 1-6 1. reacts with substances it comes into contact with (including flesh) 2. use blue litmus paper (or pH paper/pH meter) 3. H2SO4, HNO3, Ca(OH)2, NH3(aq) 4. It indicates, by its color, whether a solution is acidic or alkaline. 5. a) weakly alkaline (b) weakly acidic (c) neutral (d) strongly acidic (e) strongly alkaline (f) more acidic than b but not strongly acidic 6. green - sugar is a neutral substance Chem. Unit 11.2 page 151 1-6 1. HCl (aq) → H+(aq) + Cl- (aq) 2. 2. Their solutions contain hydrogen ions. 3. a) The solution contains fewer ions. (b) The solution contains fewer hydrogen ions. (It is a weaker acid.) 4. They contain hydroxide ions. 5. NH3 (aq) → NH4 + (aq) + OH2 (aq) 6. a) The solution contains fewer ions. b The solution contains fewer hydroxide ions. Chem. Unit 11.3 page 153 1-6 1. a) zinc + sulfuric acid → zinc sulfate + hydrogen b) sodium carbonate + sulfuric acid → sodium sulfate + water + carbon dioxide 2. ‘a’ 3. calcium chloride, Ca2+, Cl4. Use this reaction: zinc oxide + nitric acid → zinc nitrate + water 5. a) Both reactions produce calcium chloride and water. b) Only the reaction with the carbonate produces carbon dioxide. 6. a) It is a base. b) water (since neutralization takes place). Chem. Unit 11.4 page 155 1-7 1. a) It is an equation showing only the ions involved in the reaction. b) H+ (aq) + OH- (aq) → H2O (l) 2. ions that are present in the solution but not involved in the reaction 3 The hydrogen atom contains 1 proton and 1 electron (no neutron). When it becomes an ion it loses the electron, leaving just a proton. 4 a) They transfer a hydrogen ion to a base. b) They accept a hydrogen ion from an acid. 5 During neutralization a proton is transferred, but no electrons. 6 i. H+ (aq), Cl- (aq), Mg2+ (aq), O2- (aq) → Mg2+ (aq), Cl- (aq) ii. The spectator ions are Mg2+ (aq) and Cl- (aq). iii. 2H+ (aq) + O2- (s) → H2O (l) 7. 2H+ (aq) + CO3 2- (aq) → H2O (l) + CO2 (g) Chem. Unit 11.5 page 157 1-6 1. Show that it reacts with an acid to form a salt. 2. basic, acidic 3. phosphorus, sulfur, carbon (most reactive first) 4. It will turn red. 5. It will react with both acids and bases; zinc and aluminum oxides 6. Test the solution using Universal indicator paper. The paper will go green. Or use a pH meter, which will show a pH of 7. Chem. Unit 11.6 page 159 1-6 1. zinc / zinc oxide / zinc carbonate and hydrochloric acid 2. a) reaction too slow b Use lead oxide / carbonate and nitric acid. 3. more than is needed to react with the acid (so some zinc will remain unreacted) 4. to find the exact volumes of acid and alkali that react together in a neutralization reaction 5. These give more accurate volume measurements. 6. ammonia solution and nitric acid Chem. Checkup Chapter 11 Core 1. The correct choices are, in order: bases, sulfates, metals, hydrogen, carbonates, carbon dioxide, good, red, colorless, pH number, lower. 2. a) B (b) carbon dioxide (c) As A and B react together, a soluble salt forms. 3. a) i) copper(II) oxide (ii) calcium oxide (iii) phosphorus pentoxide (b) The paper turns blue. (c) ammonia (d i) copper(II) oxide + sulfuric acid → copper(II) sulfate + water (ii) neutralization (e) sulfur dioxide, sulfur trioxide, nitrogen dioxide 4 i) The missing chemicals are, in order: (b) zinc chloride, hydrogen (c) sulfuric acid (d) hydrochloric acid, sodium carbonate, carbon dioxide (e) sulfuric acid, iron, hydrogen (f) alkali, sodium nitrate, water (g) sulfuric acid, water h carbonate, sulfuric acid, copper(II) carbonate, water ii a) Ca(OH)2 (aq) + 2HNO3 (aq) → Ca(NO3)2 (aq) + 2H2O (l) b) Zn (s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g) c) 2KOH (aq) + H2SO4 (aq) → K2SO4 (aq) + 2H2O (l) d) Na2CO3 (s) + 2HCl (aq) → 2NaCl (aq) + H2O (l)+ CO2 (g) e) Fe (s) + H2SO4 (aq)→ FeSO4 (aq) + H2 (g) f )NaOH (aq) + HNO3 (aq) → NaNO3 (aq) + H2O (l) g) CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l) h) CuCO3 (s) + H2SO4 (aq)→ CuSO4 (aq) + H2O (l)+ CO2 (g) 5 a) carbon dioxide (b) i copper(II) carbonate + ethanoic acid → copper(II) ethanoate + water (ii) No more gas bubbles off, and solid remains in the beaker. (c i) copper(II) carbonate; after reaction, some remains in the beaker (ii) ethanoic acid (d) to make sure the reaction is quick and complete (e) copper(II) carbonate (f) • put some ethanoic acid into a beaker • add a spatula measure of copper(II) carbonate and stir • continue to add copper(II) carbonate until no further reaction • filter the mixture into an evaporating basin to remove unreacted copper(II) carbonate • put the evaporating basin on a tripod and gauze and evaporate some of the water (to obtain a saturated solution) • leave to cool and crystallise • remove the crystals by filtering or decanting • wash and dry the crystals (g) copper(II) oxide or hydroxide Extended 6 a) sulfuric acid (b) MgO (s) + H2SO4 (aq) → MgSO4 (aq) + H2O (l) (c) i) strong (ii) the hydrogen ion, H+ d i) a chemical that neutralizes an acid to form a salt and water ii) O2- + 2H+→ H2O 7. i) e.g. sodium chloride (or any other soluble chloride) and silver nitrate (ii) precipitation (b i) Ag+ (aq) + Cl- (aq) → AgCl(s) ii) ions: sodium (or other metal chosen), nitrate 8 a) contains water of crystallization b) Na2CO3 (aq) + 2HCl (aq) → 2NaCl (aq) + H2O (l) + CO2 (g) c) 0.014 moles d) 0.007 moles e) 0.742 g f) 1.258 g g) 0.07 moles h) 10 moles i) Na2CO3 10H2O